Trypanosoma cruzi P21 recombinant protein modulates Toxoplasma gondii infection in different experimental models of the human maternal-fetal interface
- PMID: 37915581
- PMCID: PMC10617204
- DOI: 10.3389/fimmu.2023.1243480
Trypanosoma cruzi P21 recombinant protein modulates Toxoplasma gondii infection in different experimental models of the human maternal-fetal interface
Abstract
Introduction: Toxoplasma gondii is the etiologic agent of toxoplasmosis, a disease that affects about one-third of the human population. Most infected individuals are asymptomatic, but severe cases can occur such as in congenital transmission, which can be aggravated in individuals infected with other pathogens, such as HIV-positive pregnant women. However, it is unknown whether infection by other pathogens, such as Trypanosoma cruzi, the etiologic agent of Chagas disease, as well as one of its proteins, P21, could aggravate T. gondii infection.
Methods: In this sense, we aimed to investigate the impact of T. cruzi and recombinant P21 (rP21) on T. gondii infection in BeWo cells and human placental explants.
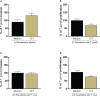
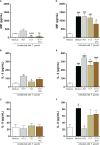
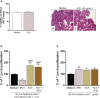
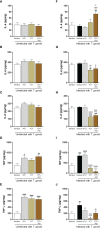
Results: Our results showed that T. cruzi infection, as well as rP21, increases invasion and decreases intracellular proliferation of T. gondii in BeWo cells. The increase in invasion promoted by rP21 is dependent on its binding to CXCR4 and the actin cytoskeleton polymerization, while the decrease in proliferation is due to an arrest in the S/M phase in the parasite cell cycle, as well as interleukin (IL)-6 upregulation and IL-8 downmodulation. On the other hand, in human placental villi, rP21 can either increase or decrease T. gondii proliferation, whereas T. cruzi infection increases T. gondii proliferation. This increase can be explained by the induction of an anti-inflammatory environment through an increase in IL-4 and a decrease in IL-6, IL-8, macrophage migration inhibitory factor (MIF), and tumor necrosis factor (TNF)-α production.
Discussion: In conclusion, in situations of coinfection, the presence of T. cruzi may favor the congenital transmission of T. gondii, highlighting the importance of neonatal screening for both diseases, as well as the importance of studies with P21 as a future therapeutic target for the treatment of Chagas disease, since it can also favor T. gondii infection.
Keywords: P21 protein; Toxoplasma gondii; Trypanosoma cruzi; coinfection; congenital toxoplasmosis; maternal-fetal interface.
Copyright © 2023 de Souza, Teixeira, Fajardo Martínez, Silva, Luz, Lima Júnior, Rosini, dos Santos, de Oliveira, Paschoalino, Barbosa, Alves, Gomes, da Silva, Ferro and Barbosa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Molan A, Nosaka K, Hunter M, Wang W. Global status of Toxoplasma gondii infection: systematic review and prevalence snapshots. Trop BioMed (2019) 36(4):898–925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

