Elevated high-mannose N-glycans hamper endometrial decidualization
- PMID: 37915610
- PMCID: PMC10616321
- DOI: 10.1016/j.isci.2023.108170
Elevated high-mannose N-glycans hamper endometrial decidualization
Abstract
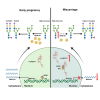
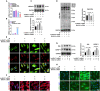
Decidualization of endometrial stromal cells is a hallmark of endometrial receptivity for embryo implantation, and dysfunctional decidualization is associated with pregnancy failure. Protein glycosylation is an important posttranslational modification that affects the structure and function of glycoproteins. Our results showed that high-mannose epitopes were elevated in the decidual tissues of miscarriage patients compared with early pregnant women by Lectin microarray. Furthermore, the level of mannosyl-oligosaccharide α-1,2 mannosidase IA (MAN1A1), a key enzyme for high-mannose glycan biosynthesis, was decreased in the decidual tissues of miscarriage patients. Screening of lncRNAs showed that lncNEAT1 level was increased in the serum and decidua of miscarriage patients, and negatively correlated with MAN1A1 expression. The results also revealed that specific binding of lncNEAT1 with nucleophosmin (NPM1)-SP1 transcription complex inhibited MAN1A1 expression and hampered endometrial decidualization and embryo implantation potential. The study suggests the new insights into the function of high-mannose glycans/MAN1A1 modification during endometrial decidualization.
Keywords: Biochemistry; Molecular biology; Physiology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells.Hum Reprod. 2022 Jun 30;37(7):1505-1524. doi: 10.1093/humrep/deac112. Hum Reprod. 2022. PMID: 35604371
-
poFUT1 promotes endometrial decidualization by enhancing the O-fucosylation of Notch1.EBioMedicine. 2019 Jun;44:563-573. doi: 10.1016/j.ebiom.2019.05.027. Epub 2019 Jun 11. EBioMedicine. 2019. PMID: 31201143 Free PMC article.
-
O-Fucosylation of BMP1 promotes endometrial decidualization by activating BMP/Smad signaling pathway.Biol Reprod. 2023 Aug 10;109(2):172-183. doi: 10.1093/biolre/ioad060. Biol Reprod. 2023. PMID: 37338142
-
FoxO1 is a cell-specific core transcription factor for endometrial remodeling and homeostasis during menstrual cycle and early pregnancy.Hum Reprod Update. 2021 Apr 21;27(3):570-583. doi: 10.1093/humupd/dmaa060. Hum Reprod Update. 2021. PMID: 33434267 Review.
-
Genome-wide analysis of histone modifications that underlie the dynamic changes in gene expression during decidualization in human endometrial stromal cells.Mol Hum Reprod. 2023 Jun 30;29(7):gaad019. doi: 10.1093/molehr/gaad019. Mol Hum Reprod. 2023. PMID: 37310913 Review.
Cited by
-
The Sweet Relationship between the Endometrium and Protein Glycosylation.Biomolecules. 2024 Jun 27;14(7):770. doi: 10.3390/biom14070770. Biomolecules. 2024. PMID: 39062484 Free PMC article. Review.
-
Unveiling sialoglycans' immune mastery in pregnancy and their intersection with tumor biology.Front Immunol. 2024 Dec 20;15:1479181. doi: 10.3389/fimmu.2024.1479181. eCollection 2024. Front Immunol. 2024. PMID: 39759524 Free PMC article. Review.
References
-
- Lidegaard Ø., Mikkelsen A.P., Egerup P., Kolte A.M., Rasmussen S.C., Nielsen H.S. Pregnancy loss: A 40-year nationwide assessment. Acta Obstet. Gynecol. Scand. 2020;99:1492–1496. - PubMed
-
- Ramhorst R., Grasso E., Vota D., Gori S., Hauk V., Paparini D., Calo G., Pérez Leirós C. From decidualization to pregnancy progression: An overview of immune and metabolic effects of VIP. Am. J. Reprod. Immunol. 2022;88:e13601. - PubMed
LinkOut - more resources
Full Text Sources

