Arachidonic acid activates NLRP3 inflammasome in MDSCs via FATP2 to promote post-transplant tumour recurrence in steatotic liver grafts
- PMID: 37916155
- PMCID: PMC10616418
- DOI: 10.1016/j.jhepr.2023.100895
Arachidonic acid activates NLRP3 inflammasome in MDSCs via FATP2 to promote post-transplant tumour recurrence in steatotic liver grafts
Abstract
Background & aims: The steatotic grafts have been applied in liver transplantation frequently owing to the high incidence of non-alcoholic fatty liver disease. However, fatty livers are vulnerable to graft injury. Myeloid-derived suppressor cell (MDSC) recruitment during liver graft injury promotes tumour recurrence. Lipid metabolism exerts the immunological influence on MDSCs in tumour progression. Here, we aimed to explore the role and mechanism of inflammasome activation in MDSCs induced by lipid metabolism during fatty liver graft injury and the subsequent effects on tumour recurrence.
Methods: MDSC populations and nucleotide-binding oligomerisation domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome levels were investigated in a clinical cohort and a rat liver transplantation model. The mechanism of NLRP3 activation by specific fatty acids was explored in mouse hepatic ischaemia/reperfusion injury (IRI) with tumour recurrence model and in vitro studies.
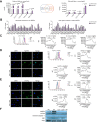
Results: MDSC populations and NLRP3 levels were increased with higher tumour recurrent rate in patients using steatotic grafts. NLRP3 was upregulated in MDSCs with lipid accumulation post mouse fatty liver IRI. Mechanistically, arachidonic acid was discovered to activate NLRP3 inflammasome in MDSCs through fatty acid transport protein 2 (FATP2), which was identified by screening lipid uptake receptors. The mitochondrial dysfunction with enhanced reactive oxygen species bridged arachidonic acid uptake and NLRP3 activation in MDSCs, which subsequently stimulated CD4+ T cells producing more IL-17 in fatty liver IRI. Blockade of FATP2 inhibited NLRP3 activation in MDSCs, IL-17 production in CD4+ T cells, and the tumour recurrence post fatty liver IRI.
Conclusions: During fatty liver graft injury, arachidonic acid activated NLRP3 inflammasome in MDSCs through FATP2, which subsequently stimulated CD4+ T cells producing IL-17 to promote tumour recurrence post transplantation.
Impact and implications: The high incidence of non-alcoholic fatty liver disease resulted in the frequent application of steatotic donors in liver transplantation. Our data showed that the patients who underwent liver transplantation using fatty grafts experienced higher tumour recurrence. We found that arachidonic acid activated NLRP3 inflammasome in MDSCs through FATP2 during fatty liver graft injury, which led to more IL-17 secretion of CD4+ T cells and promoted tumour recurrence post transplantation. The inflammasome activation by aberrant fatty acid metabolism in MDSCs bridged the acute-phase fatty liver graft injury and liver tumour recurrence.
Keywords: Inflammasome; Lipid metabolism; MDSC; Steatotic liver graft; Tumour recurrence.
© 2023 The Authors.
Conflict of interest statement
All authors have no conflicts of interest to be declared. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures







References
-
- Younossi Z., Tacke F., Arrese M., Chander Sharma B., Mostafa I., Bugianesi E., et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–2682. - PubMed
-
- Linares I., Hamar M., Selzner N., Selzner M. Steatosis in liver transplantation: current limitations and future strategies. Transplantation. 2019;103:78–90. - PubMed
-
- Liu J., Pang L., Ng K.T.P., Chiu T.L.S., Liu H., Liu X., et al. Compromised AMPK-PGC1α axis exacerbated steatotic graft injury by dysregulating mitochondrial homeostasis in living donor liver transplantation. Ann Surg. 2022;276:e483–e492. - PubMed
-
- Orci L., Lacotte S., Oldani G., Slits F., De Vito C., Crowe L., et al. Effect of ischaemic preconditioning on recurrence of hepatocellular carcinoma in an experimental model of liver steatosis. Br J Surg. 2016;103:417–426. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

