Driving electrochemical reactions at the microscale using CMOS microelectrode arrays
- PMID: 37916299
- PMCID: PMC10661664
- DOI: 10.1039/d3lc00630a
Driving electrochemical reactions at the microscale using CMOS microelectrode arrays
Abstract
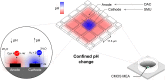
Precise control of pH values at electrode interfaces enables the systematic investigation of pH-dependent processes by electrochemical means. In this work, we employed high-density complementary metal-oxide-semiconductor (CMOS) microelectrode arrays (MEAs) as miniaturized systems to induce and confine electrochemical reactions in areas corresponding to the pitch of single electrodes (17.5 μm). First, we present a strategy for generating localized pH patterns on the surface of the CMOS MEA with unprecedented spatial resolution. Leveraging the versatile routing capabilities of the switch matrix beneath the CMOS MEA, we created arbitrary combinations of anodic and cathodic electrodes and hence pH patterns. Moreover, we utilized the system to produce polymeric surface patterns by additive and subtractive methods. For additive patterning, we controlled the in situ formation of polydopamine at the microelectrode surface through oxidation of free dopamine above a threshold pH > 8.5. For subtractive patterning, we removed cell-adhesive poly-L-lysine from the electrode surface and backfilled the voids with antifouling polymers. Such polymers were chosen to provide a proof-of-concept application of controlling neuronal growth via electrochemically-induced patterns on the CMOS MEA surface. Importantly, our platform is compatible with commercially available high-density MEAs and requires no custom equipment, rendering the findings generalizable and accessible.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures





References
-
- Fiedler S. Hagedorn R. Schnelle T. Richter E. Wagner B. Fuhr G. Anal. Chem. 1995;67:820–828. doi: 10.1021/ac00101a006. - DOI
LinkOut - more resources
Full Text Sources

