A Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy
- PMID: 37916620
- PMCID: PMC7615905
- DOI: 10.1056/NEJMoa2305635
A Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy
Abstract
Background: A proliferation-inducing ligand (APRIL) is implicated in the pathogenesis of IgA nephropathy. Sibeprenlimab is a humanized IgG2 monoclonal antibody that binds to and neutralizes APRIL.
Methods: In this phase 2, multicenter, double-blind, randomized, placebo-controlled, parallel-group trial, we randomly assigned adults with biopsy-confirmed IgA nephropathy who were at high risk for disease progression, despite having received standard-care treatment, in a 1:1:1:1 ratio to receive intravenous sibeprenlimab at a dose of 2, 4, or 8 mg per kilogram of body weight or placebo once monthly for 12 months. The primary end point was the change from baseline in the log-transformed 24-hour urinary protein-to-creatinine ratio at month 12. Secondary end points included the change from baseline in the estimated glomerular filtration rate (eGFR) at month 12. Safety was also assessed.
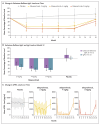
Results: Among 155 patients who underwent randomization, 38 received sibeprenlimab at a dose of 2 mg per kilogram, 41 received sibeprenlimab at a dose of 4 mg per kilogram, 38 received sibeprenlimab at a dose of 8 mg per kilogram, and 38 received placebo. At 12 months, the geometric mean ratio reduction (±SE) from baseline in the 24-hour urinary protein-to-creatinine ratio was 47.2±8.2%, 58.8±6.1%, 62.0±5.7%, and 20.0±12.6% in the sibeprenlimab 2-mg, 4-mg, and 8-mg groups and the placebo group, respectively. At 12 months, the least-squares mean (±SE) change from baseline in eGFR was -2.7±1.8, 0.2±1.7, -1.5±1.8, and -7.4±1.8 ml per minute per 1.73 m2 in the sibeprenlimab 2-mg, 4-mg, and 8-mg groups and the placebo group, respectively. The incidence of adverse events that occurred after the start of administration of sibeprenlimab or placebo was 78.6% in the pooled sibeprenlimab groups and 71.1% in the placebo group.
Conclusions: In patients with IgA nephropathy, 12 months of treatment with sibeprenlimab resulted in a significantly greater decrease in proteinuria than placebo. (Funded by Visterra; ENVISION ClinicalTrials.gov number, NCT04287985; EudraCT number, 2019-002531-29.).
Copyright © 2023 Massachusetts Medical Society.
Figures

Comment in
-
Targeting APRIL to slow progression of IgA nephropathy.Nat Rev Nephrol. 2024 Jan;20(1):6. doi: 10.1038/s41581-023-00794-x. Nat Rev Nephrol. 2024. PMID: 38012265 No abstract available.
-
Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy.N Engl J Med. 2024 Apr 4;390(13):1245. doi: 10.1056/NEJMc2401277. N Engl J Med. 2024. PMID: 38598587 No abstract available.
-
Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy. Reply.N Engl J Med. 2024 Apr 4;390(13):1245-1246. doi: 10.1056/NEJMc2401277. N Engl J Med. 2024. PMID: 38598588 No abstract available.
References
-
- Lai KN, Tang SCW, Schena FP, et al. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–S276. - PubMed
-
- Lafayette R, Kristensen J, Stone A, et al. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (Nef IgArd): 2-year results from a randomised phase 3 trial. Lancet. 2023;402:859–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
