Disruption of trehalose periplasmic recycling dysregulates cAMP-CRP signaling in Escherichia coli during stationary phase
- PMID: 37916804
- PMCID: PMC10662143
- DOI: 10.1128/jb.00292-23
Disruption of trehalose periplasmic recycling dysregulates cAMP-CRP signaling in Escherichia coli during stationary phase
Abstract
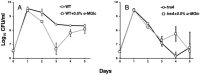
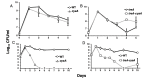
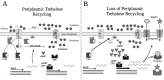
Survival during starvation hinges on the ability to manage intracellular energy reserves and to initiate appropriate metabolic responses to perturbations of such reserves. How Escherichia coli manage carbon storage systems under starvation stress, as well as transpose changes in intracellular metabolite levels into regulatory signals, is not well understood. Endogenous trehalose metabolism may be at the center of these processes, coupling carbon storage with carbon starvation responses. The coupled transport to the periplasm and subsequent hydrolysis of trehalose back to glucose for transport to the cytoplasm may function as a crucial metabolic signaling pathway. Although trehalose has been characterized as a stress protectant in E. coli, the disaccharide also functions as both an energy storage compound and a regulator of carbohydrate metabolism in fungi, plants, and other bacteria. Our research explores the metabolic regulatory properties of trehalose in E. coli and a potential mechanism by which the intracellular carbon pool is interconnected with regulatory circuits, enabling long-term survival.
Keywords: carbohydrate metabolism; long-term survival; stationary phase; trehalose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

