Modulation of Gene Expression in the Eye with Antisense Oligonucleotides
- PMID: 37917066
- PMCID: PMC10698777
- DOI: 10.1089/nat.2023.0044
Modulation of Gene Expression in the Eye with Antisense Oligonucleotides
Abstract
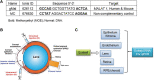
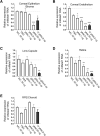
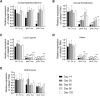
One advantage of antisense oligonucleotides (ASOs) for drug development is their long-lasting gene knockdown after administration in vivo. In this study, we examine the effect on gene expression after intraocular injection in target tissues in the eye. We examined expression levels of the Malat1 gene after intracameral or intravitreal (IV) injection of an anti-Malat1 ASO in corneal epithelium/stroma, corneal endothelium, lens capsule epithelium, neurosensory retina, and retinal pigment epithelium/choroid of the mouse eye. We assessed potency of the compound at 7 days as well as duration of the gene knockdown at 14, 28, 60, 90, and 120 days. The ASO was more potent when delivered by IV injection relative to intracameral injection, regardless of whether the tissues analyzed were at the front or back of the eye. For corneal endothelium, inhibition was >50% after 120 days for ASO at 50 μg. At IV dosages of 6 μg, we observed >75% inhibition of gene expression in the retina and lens epithelium for up to 120 days. ASOs have potential as long-lasting gene knockdown agents in the mouse eye, but efficacy varies depending on the specific ocular target tissue and injection protocol.
Keywords: antisense oligonucleotide; cornea; intraocular; retina.
Conflict of interest statement
T.P.P. and F.R. are employees of Ionis Pharmaceuticals. V.V.M., D.R.C., and J.H. hold a patent related to the use of ASOs to treat Fuchs' Dystrophy.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

