A Mini Review of the Impact of Baseline Disease Severity on Clinical Outcomes: Should We Compare Atopic Dermatitis Clinical Trials?
- PMID: 37917285
- PMCID: PMC10689679
- DOI: 10.1007/s13555-023-01052-5
A Mini Review of the Impact of Baseline Disease Severity on Clinical Outcomes: Should We Compare Atopic Dermatitis Clinical Trials?
Abstract
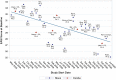
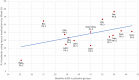
Based on clinical trials of systemic treatments in adults with moderate-to-severe atopic dermatitis (AD) reported between 2014 and 2023, we used linear regression to investigate relationships between baseline Eczema Area and Severity Index (EASI) scores and (1) study start date, (2) EASI response, and (3) rescue medication rates. Analysis 1 was conducted with all patients from monotherapy and combination therapy trials; analyses 2 and 3 used monotherapy trial placebo arms. Across 32 trials with a baseline inclusion criterion of EASI ≥ 16, baseline mean EASI scores decreased with study start date. The lowest and highest baseline mean EASI scores were 25.1 and 33.6 (median 21.1 and 30.5), reported for the WW001 Phase 2 trial of rademikibart (formerly CBP-201; start date, July 2020) and the SOLO1 Phase 3 trial of dupilumab (start date, December 2014), respectively. In placebo arms, lower baseline EASI scores tended to be associated with greater percent reductions in EASI scores at Week 16 and less rescue medication usage. The WW001 trial placebo arm had the lowest baseline EASI score (mean 25.2; median 22.1), lowest rescue medication rate (14.3%), and a large reduction in least squares mean EASI scores (- 39.7%) at Week 16. In summary, baseline mean EASI scores have decreased across clinical trials conducted during the last decade. Less severe AD at baseline tended to be associated with greater placebo response and less use of rescue medications in placebo arms. Intertrial differences in variables, such as baseline AD severity, limit the validity of indirectly comparing clinical trials.
Keywords: Atopic dermatitis; Baseline severity; CBP-201; Clinical trial comparison; IL-13; IL-4; JAK inhibitor; Placebo response; Rademikibart.
© 2023. The Author(s).
Conflict of interest statement
Jonathan I. Silverberg has acted as an advisor, speaker, or consultant for AbbVie Inc., AFYX, Arena, Asana, BiomX, Bluefin, Bodewell, Boehringer Ingelheim, Celgene Corporation, Connect Biopharma, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Hoth, Incyte, Kiniksa, Leo Pharma, Luna, Menlo Therapeutics, Novartis, Pfizer, RAPT, Regeneron Pharmaceuticals, and Sanofi. Researcher for Galderma. Selwyn Ho and Raúl Collazo are either current or former employees or shareholders of Connect Biopharma. Selwyn Ho is now an employee of Medigene AG, Planegg/Martinsried, Germany.
Figures

References
-
- Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387:40–52. doi: 10.1016/S0140-6736(15)00388-8. - DOI - PubMed
-
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, Paller AS, Armstrong AW, Drew J, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411. doi: 10.1001/jamadermatol.2020.0079. - DOI - PMC - PubMed
-
- de Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical t. Br J Dermatol. 2018;178:1083–1101. doi: 10.1111/bjd.16156. - DOI - PubMed
-
- Simpson EL, Lacour J-P, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242–255. doi: 10.1111/bjd.18898. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

