Treatment of diabetic kidney disease. A network meta-analysis
- PMID: 37917640
- PMCID: PMC10621862
- DOI: 10.1371/journal.pone.0293183
Treatment of diabetic kidney disease. A network meta-analysis
Abstract
Background: Diabetic kidney disease (DKD) is a health burden of rising importance. Slowing progression to end stage kidney disease is the main goal of drug treatment. The aim of this analysis is to compare drug treatments of DKD by means of a systemic review and a network meta-analysis.
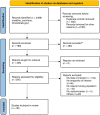
Methods: We searched Medline, CENTRAL and clinicaltrials.gov for randomized, controlled studies including adults with DKD treated with the following drugs of interest: single angiotensin-converting-enzyme-inhibitor or angiotensin-receptor-blocker (single ACEi/ARB), angiotensin-converting-enzyme-inhibitor and angiotensin-receptor-blocker combination (ACEi+ARB combination), aldosterone antagonists, direct renin inhibitors, non-steroidal mineralocorticoid-receptor-antagonists (nsMRA) and sodium-glucose cotransporter-2 inhibitors (SGLT2i). As primary endpoints, we defined: overall mortality and end-stage kidney disease, as secondary endpoints: renal composite outcome and albuminuria and as safety endpoints: acute kidney injury, hyperkalemia and hypotension. Under the use of a random effects model, we computed the overall effect estimates using the statistic program R4.1 and the corresponding package "netmeta". Risk of bias was assessed using the RoB 2 tool and the quality of evidence of each pairwise comparison was rated according to GRADE (Grading of Recommendations Assessment, Development and Evaluation).
Results: Of initial 3489 publications, 38 clinical trials were found eligible, in total including 42346 patients. Concerning the primary endpoints overall mortality and end stage kidney disease, SGLT2i on top of single ACEi/ARB compared to single ACEi/ARB was the only intervention significantly reducing the odds of mortality (OR 0.81, 95%CI 0.70-0.95) and end-stage kidney disease (OR 0.69, 95%CI 0.54-0.88). The indirect comparison of nsMRA vs SGLT2i in our composite endpoint suggests a superiority of SGLT2i (OR 0.60, 95%CI 0.47-0.76). Concerning safety endpoints, nsMRA and SGLT2i showed benefits compared to the others.
Conclusions: As the only drug class, SGLT2i showed in our analysis beneficial effects on top of ACEi/ARB treatment regarding mortality and end stage kidney disease and by that reconfirmed its position as treatment option for diabetic kidney disease. nsMRA reduced the odds for a combined renal endpoint and did not raise any safety concerns, justifying its application.
Copyright: © 2023 Büttner et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al.. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9 - DOI - PMC - PubMed
-
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Vol. 120, Nephron: —Clinical Practice. 2012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

