Lenacapavir: A Novel, Potent, and Selective First-in-Class Inhibitor of HIV-1 Capsid Function Exhibits Optimal Pharmacokinetic Properties for a Long-Acting Injectable Antiretroviral Agent
- PMID: 37917742
- PMCID: PMC10698746
- DOI: 10.1021/acs.molpharmaceut.3c00626
Lenacapavir: A Novel, Potent, and Selective First-in-Class Inhibitor of HIV-1 Capsid Function Exhibits Optimal Pharmacokinetic Properties for a Long-Acting Injectable Antiretroviral Agent
Abstract
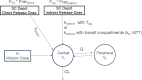
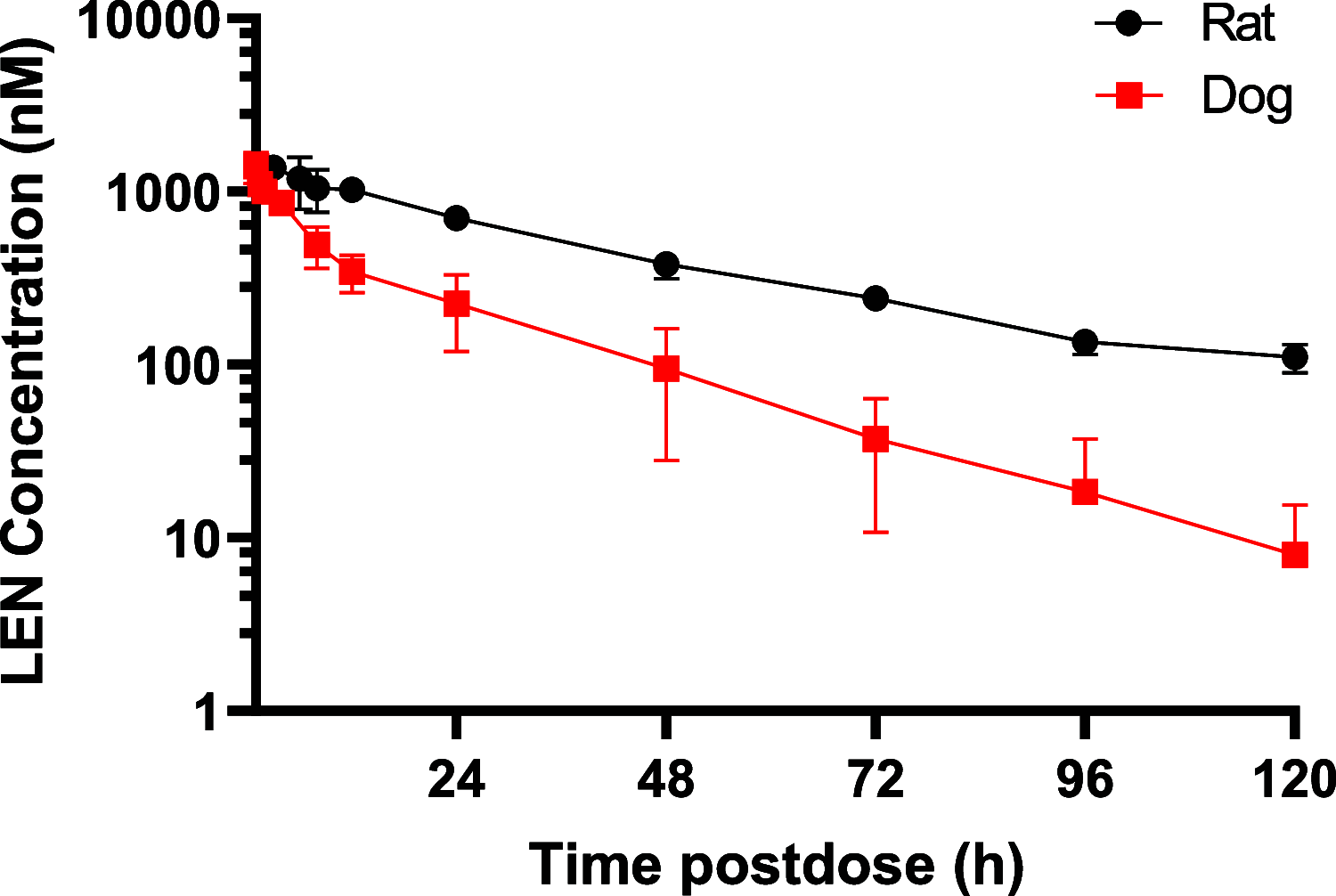
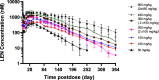
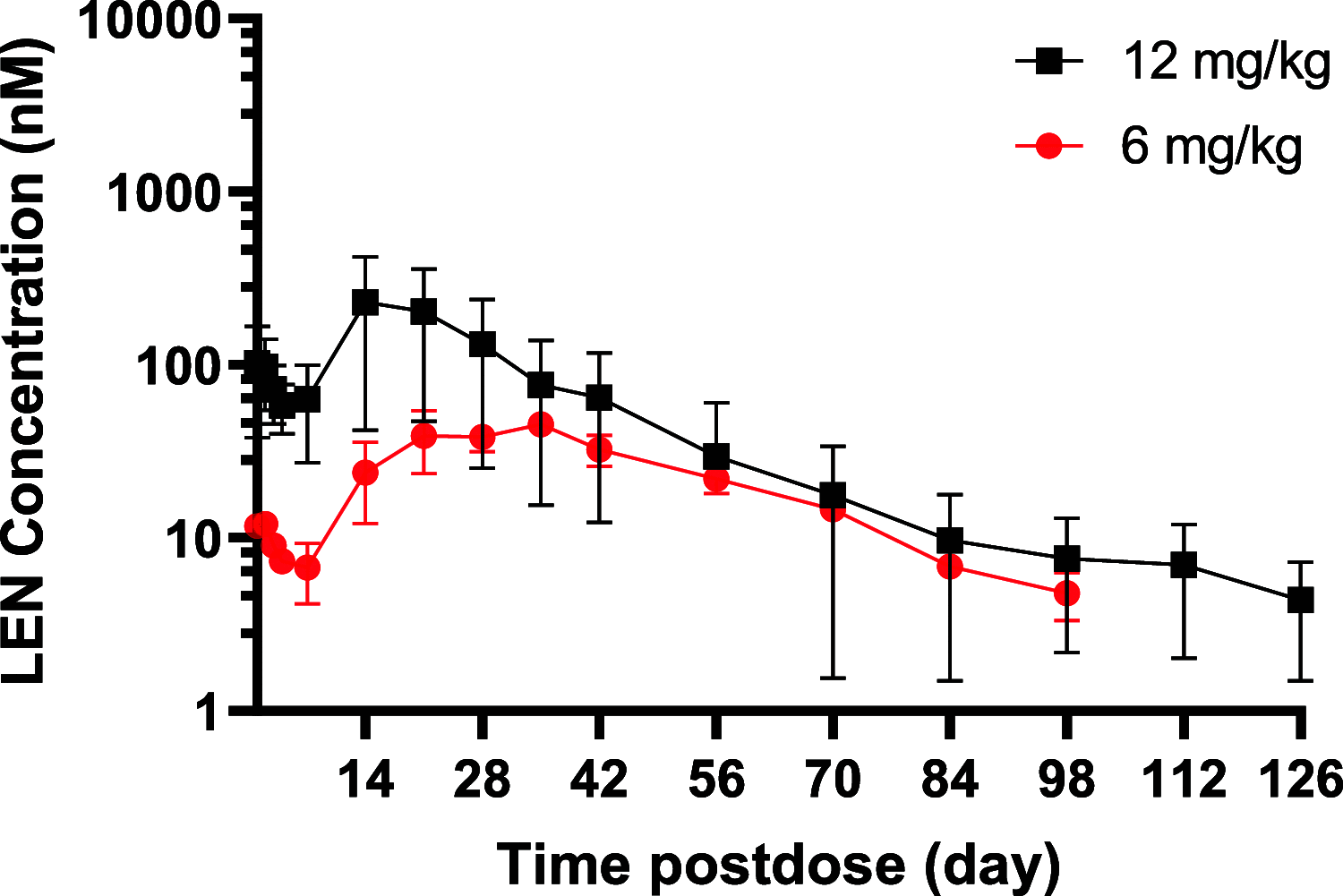
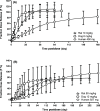
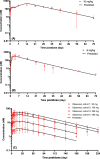
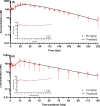
Lenacapavir (LEN) is a picomolar first-in-class capsid inhibitor of human immunodeficiency virus type 1 (HIV-1) with a multistage mechanism of action and no known cross resistance to other existing antiretroviral (ARV) drug classes. LEN exhibits a low aqueous solubility and exceptionally low systemic clearance following intravenous (IV) administration in nonclinical species and humans. LEN formulated in an aqueous suspension or a PEG/water solution formulation showed sustained plasma exposure levels with no unintended rapid drug release following subcutaneous (SC) administration to rats and dogs. A high total fraction dose release was observed with both formulations. The long-acting pharmacokinetics (PK) were recapitulated in humans following SC administration of both formulations. The SC PK profiles displayed two-phase absorption kinetics in both animals and humans with an initial fast-release absorption phase, followed by a slow-release absorption phase. Noncompartmental and compartmental analyses informed the LEN systemic input rate from the SC depot and exit rate from the body. Modeling-enabled deconvolution of the input rates from two processes: absorption of the soluble fraction (minor) from a direct fast-release process leading to the early PK phase and absorption of the precipitated fraction (major) from an indirect slow-release process leading to the later PK phase. LEN SC PK showed flip-flop kinetics due to the input rate being substantially slower than the systemic exit rate. LEN input rates via the slow-release process in humans were slower than those in both rats and dogs. Overall, the combination of high potency, exceptional stability, and optimal release rate from the injection depot make LEN well suited for a parenteral long-acting formulation that can be administered once up to every 6 months in humans for the prevention and treatment of HIV-1.
Keywords: HIV-1 capsid inhibitor; PK modeling; combination antiretroviral therapy; human PK; long-acting injectable; nonclinical pharmacokinetics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Li W.; Tang J.; Lee D.; Tice T. R.; Schwendeman S. P.; Prausnitz M. R. Clinical translation of long-acting drug delivery formulations. Nat. Rev. Mater. 2022, 7 (5), 406–420. 10.1038/s41578-021-00405-w. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

