Synergistic Role of Water and Oxygen Leads to Degradation in Formamidinium-Based Halide Perovskites
- PMID: 37917967
- PMCID: PMC10655111
- DOI: 10.1021/jacs.3c05657
Synergistic Role of Water and Oxygen Leads to Degradation in Formamidinium-Based Halide Perovskites
Abstract
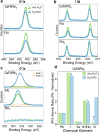
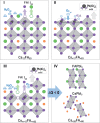
Mixed-cation metal halide perovskites have shown remarkable progress in photovoltaic applications with high power conversion efficiencies. However, to achieve large-scale deployment of this technology, efficiencies must be complemented by long-term durability. The latter is limited by external factors, such as exposure to humidity and air, which lead to the rapid degradation of the perovskite materials and devices. In this work, we study the mechanisms causing Cs and formamidinium (FA)-based halide perovskite phase transformations and stabilization during moisture and air exposure. We use in situ X-ray scattering, X-ray photoelectron spectroscopy, and first-principles calculations to study these chemical interactions and their effects on structure. We unravel a surface reaction pathway involving the dissolution of FAI by water and iodide oxidation by oxygen, driving the Cs/FA ratio into thermodynamically unstable regions, leading to undesirable phase transformations. This work demonstrates the interplay of bulk phase transformations with surface chemical reactions, providing a detailed understanding of the degradation mechanism and strategies for designing durable and efficient perovskite materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- An Y.; Hidalgo J.; Perini C. A. R.; Castro-Méndez A. F.; Vagott J. N.; Bairley K.; Wang S.; Li X.; Correa-Baena J. P. Structural Stability of Formamidinium- And Cesium-Based Halide Perovskites. ACS Energy Lett. 2021, 6 (5), 1942–1969. 10.1021/acsenergylett.1c00354. - DOI
-
- Charles B.; Weller M. T.; Rieger S.; Hatcher L. E.; Henry P. F.; Feldmann J.; Wolverson D.; Wilson C. C. Phase Behavior and Substitution Limit of Mixed Cesium-Formamidinium Lead Triiodide Perovskites. Chem. Mater. 2020, 32 (6), 2282–2291. 10.1021/acs.chemmater.9b04032. - DOI
-
- Li Z.; Yang M.; Park J. S.; Wei S. H.; Berry J. J.; Zhu K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28 (1), 284–292. 10.1021/acs.chemmater.5b04107. - DOI
-
- Weber O. J.; Ghosh D.; Gaines S.; Henry P. F.; Walker A. B.; Islam M. S.; Weller M. T. Phase Behavior and Polymorphism of Formamidinium Lead Iodide. Chem. Mater. 2018, 30 (11), 3768–3778. 10.1021/acs.chemmater.8b00862. - DOI
LinkOut - more resources
Full Text Sources

