The proteomic landscape of synaptic diversity across brain regions and cell types
- PMID: 37918396
- PMCID: PMC10686415
- DOI: 10.1016/j.cell.2023.09.028
The proteomic landscape of synaptic diversity across brain regions and cell types
Abstract
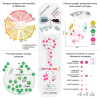
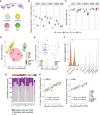
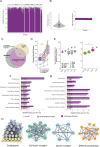
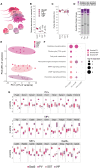
Neurons build synaptic contacts using different protein combinations that define the specificity, function, and plasticity potential of synapses; however, the diversity of synaptic proteomes remains largely unexplored. We prepared synaptosomes from 7 different transgenic mouse lines with fluorescently labeled presynaptic terminals. Combining microdissection of 5 different brain regions with fluorescent-activated synaptosome sorting (FASS), we isolated and analyzed the proteomes of 18 different synapse types. We discovered ∼1,800 unique synapse-type-enriched proteins and allocated thousands of proteins to different types of synapses (https://syndive.org/). We identify shared synaptic protein modules and highlight the proteomic hotspots for synapse specialization. We reveal unique and common features of the striatal dopaminergic proteome and discover the proteome signatures that relate to the functional properties of different interneuron classes. This study provides a molecular systems-biology analysis of synapses and a framework to integrate proteomic information for synapse subtypes of interest with cellular or circuit-level experiments.
Keywords: dopaminergic synapses; excitatory synapses; fluorescence-activated synaptosome sorting; inhibitory synapses; proteomics; synapse; synapse diversity; synaptic proteins; synaptic proteomics.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures










References
-
- Hanus C., Schuman E.M. Proteostasis in complex dendrites. Nat. Rev. Neurosci. 2013;14:638–648. - PubMed
-
- Magee J.C., Grienberger C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 2020;43:95–117. - PubMed
-
- de Wit J., Ghosh A. Specification of synaptic connectivity by cell surface interactions. Nat. Rev. Neurosci. 2016;17:22–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

