Diagnostic accuracy of a three-gene Mycobacterium tuberculosis host response cartridge using fingerstick blood for childhood tuberculosis: a multicentre prospective study in low-income and middle-income countries
- PMID: 37918414
- PMCID: PMC10808504
- DOI: 10.1016/S1473-3099(23)00491-7
Diagnostic accuracy of a three-gene Mycobacterium tuberculosis host response cartridge using fingerstick blood for childhood tuberculosis: a multicentre prospective study in low-income and middle-income countries
Abstract
Background: Childhood tuberculosis remains a major cause of morbidity and mortality in part due to missed diagnosis. Diagnostic methods with enhanced sensitivity using easy-to-obtain specimens are needed. We aimed to assess the diagnostic accuracy of the Cepheid Mycobacterium tuberculosis Host Response prototype cartridge (MTB-HR), a candidate test measuring a three-gene transcriptomic signature from fingerstick blood, in children with presumptive tuberculosis disease.
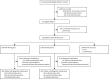
Methods: RaPaed-TB was a prospective diagnostic accuracy study conducted at four sites in African countries (Malawi, Mozambique, South Africa, and Tanzania) and one site in India. Children younger than 15 years with presumptive pulmonary or extrapulmonary tuberculosis were enrolled between Jan 21, 2019, and June 30, 2021. MTB-HR was performed at baseline and at 1 month in all children and was repeated at 3 months and 6 months in children on tuberculosis treatment. Accuracy was compared with tuberculosis status based on standardised microbiological, radiological, and clinical data.
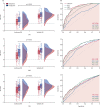
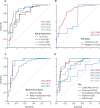
Findings: 5313 potentially eligible children were screened, of whom 975 were eligible. 784 children had MTB-HR test results, of whom 639 had a diagnostic classification and were included in the analysis. MTB-HR differentiated children with culture-confirmed tuberculosis from those with unlikely tuberculosis with a sensitivity of 59·8% (95% CI 50·8-68·4). Using any microbiological confirmation (culture, Xpert MTB/RIF Ultra, or both), sensitivity was 41·6% (34·7-48·7), and using a composite clinical reference standard, sensitivity was 29·6% (25·4-34·2). Specificity for all three reference standards was 90·3% (95% CI 85·5-94·0). Performance was similar in different age groups and by malnutrition status. Among children living with HIV, accuracy against the strict reference standard tended to be lower (sensitivity 50·0%, 15·7-84·3) compared with those without HIV (61·0%, 51·6-69·9), although the difference did not reach statistical significance. Combining baseline MTB-HR result with one Ultra result identified 71·2% of children with microbiologically confirmed tuberculosis.
Interpretation: MTB-HR showed promising diagnostic accuracy for culture-confirmed tuberculosis in this large, geographically diverse, paediatric cohort and hard-to-diagnose subgroups.
Funding: European and Developing Countries Clinical Trials Partnership, UK Medical Research Council, Swedish International Development Cooperation Agency, Bundesministerium für Bildung und Forschung; German Center for Infection Research (DZIF).
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare receiving grant funding for this work from the second European and Developing Countries Clinical Trials Partnership programme (EDCTP2), the German Center for Infection Research (DZIF), and Beckman Coulter to their respective institutions. Cepheid provided testing kits at no cost. ZF-S and HJZ declare funding from the SA-MRC Unit on Child and Adolescent Health to their institution. TDM received funding to his institution from Médicins Sans Frontières 2017–23, GOSH Charity Intramural COVID-19 Rapid Response Funding, Global Alliance Against Tuberculosis, and EU Innovative Medicines Initiative for other research activities. TDM receives personal payment in his function as Editor in Chief of Annals of Clinical Microbiology and Antimicrobials.
Figures
References
-
- WHO . World Health Organization; Geneva: 2022. Global tuberculosis report 2022.
-
- WHO . World Health Organization; Geneva: 2018. Roadmap towards ending TB in children and adolescents.
-
- Tebruegge M, Ritz N, Curtis N, Shingadia D. Diagnostic tests for childhood tuberculosis: past imperfect, present tense and future perfect? Pediatr Infect Dis J. 2015;34:1014–1019. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

