Lipid Nanoparticle-Mediated Hit-and-Run Approaches Yield Efficient and Safe In Situ Gene Editing in Human Skin
- PMID: 37918441
- PMCID: PMC10655174
- DOI: 10.1021/acsnano.3c08644
Lipid Nanoparticle-Mediated Hit-and-Run Approaches Yield Efficient and Safe In Situ Gene Editing in Human Skin
Abstract
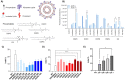
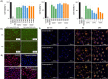
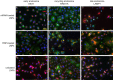
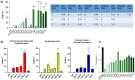
Despite exciting advances in gene editing, the efficient delivery of genetic tools to extrahepatic tissues remains challenging. This holds particularly true for the skin, which poses a highly restrictive delivery barrier. In this study, we ran a head-to-head comparison between Cas9 mRNA or ribonucleoprotein (RNP)-loaded lipid nanoparticles (LNPs) to deliver gene editing tools into epidermal layers of human skin, aiming for in situ gene editing. We observed distinct LNP composition and cell-specific effects such as an extended presence of RNP in slow-cycling epithelial cells for up to 72 h. While obtaining similar gene editing rates using Cas9 RNP and mRNA with MC3-based LNPs (10-16%), mRNA-loaded LNPs proved to be more cytotoxic. Interestingly, ionizable lipids with a pKa ∼ 7.1 yielded superior gene editing rates (55%-72%) in two-dimensional (2D) epithelial cells while no single guide RNA-dependent off-target effects were detectable. Unexpectedly, these high 2D editing efficacies did not translate to actual skin tissue where overall gene editing rates between 5%-12% were achieved after a single application and irrespective of the LNP composition. Finally, we successfully base-corrected a disease-causing mutation with an efficacy of ∼5% in autosomal recessive congenital ichthyosis patient cells, showcasing the potential of this strategy for the treatment of monogenic skin diseases. Taken together, this study demonstrates the feasibility of an in situ correction of disease-causing mutations in the skin that could provide effective treatment and potentially even a cure for rare, monogenic, and common skin diseases.
Keywords: ARCI; base editing; gene delivery; gene editing; genodermatoses; lipid nanoparticles; skin.
Conflict of interest statement
The authors declare the following competing financial interest(s): KA, DW, JK are employees of NanoVation Therapeutics. PRC has a financial interest in Acuitas Therapeutics and NanoVation Therapeutics as well as being Chair of NanoVation Therapeutics. EJ is a co-founder of NanoVation.The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

