Th17-inducing dendritic cell vaccines stimulate effective CD4 T cell-dependent antitumor immunity in ovarian cancer that overcomes resistance to immune checkpoint blockade
- PMID: 37918918
- PMCID: PMC10626769
- DOI: 10.1136/jitc-2023-007661
Th17-inducing dendritic cell vaccines stimulate effective CD4 T cell-dependent antitumor immunity in ovarian cancer that overcomes resistance to immune checkpoint blockade
Abstract
Background: Ovarian cancer (OC), a highly lethal cancer in women, has a 48% 5-year overall survival rate. Prior studies link the presence of IL-17 and Th17 T cells in the tumor microenvironment to improved survival in OC patients. To determine if Th17-inducing vaccines are therapeutically effective in OC, we created a murine model of Th17-inducing dendritic cell (DC) (Th17-DC) vaccination generated by stimulating IL-15 while blocking p38 MAPK in bone marrow-derived DCs, followed by antigen pulsing.
Methods: ID8 tumor cells were injected intraperitoneally into mice. Mice were treated with Th17-DC or conventional DC (cDC) vaccine alone or with immune checkpoint blockade (ICB). Systemic immunity, tumor associated immunity, tumor size and survival were examined using a variety of experimental strategies.
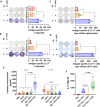
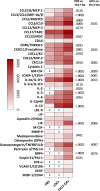
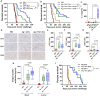
Results: Th17-DC vaccines increased Th17 T cells in the tumor microenvironment, reshaped the myeloid microenvironment, and improved mouse survival compared with cDC vaccines. ICB had limited efficacy in OC, but Th17-inducing DC vaccination sensitized it to anti-PD-1 ICB, resulting in durable progression-free survival by overcoming IL-10-mediated resistance. Th17-DC vaccine efficacy, alone or with ICB, was mediated by CD4 T cells, but not CD8 T cells.
Conclusions: These findings emphasize using biologically relevant immune modifiers, like Th17-DC vaccines, in OC treatment to reshape the tumor microenvironment and enhance clinical responses to ICB therapy.
Keywords: Antigens; CD4-Positive T-Lymphocytes; Immunomodulation; Immunotherapy, Active; Tumor Escape.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MC is an inventor on a patent filed by the University of Arkansas, entitled ‘Inhibition of dendritic cell-driven regulatory T cell activation and potentiation of tumor antigen-specific T cell responses by interleukin-15 and MAP kinase inhibitor.’ KLK and MB are inventors on a patent filed by the Mayo Clinic, entitled ‘Dendritic Cell Based Vaccines Combined with Pembrolizumab for the Treatment of Advanced Ovarian Cancer.’
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
