CDK4/6 Inhibition With Lerociclib is a Potential Therapeutic Strategy for the Treatment of Pediatric Sarcomas
- PMID: 37919169
- PMCID: PMC10922146
- DOI: 10.1016/j.jpedsurg.2023.10.004
CDK4/6 Inhibition With Lerociclib is a Potential Therapeutic Strategy for the Treatment of Pediatric Sarcomas
Abstract
Background: Sarcomas are a heterogenous collection of bone and soft tissue tumors. The heterogeneity of these tumors makes it difficult to standardize treatment. CDK 4/6 inhibitors are a family of targeted agents which limit cell cycle progression and have been shown to be upregulated in sarcomas. In the current preclinical study, we evaluated the effects of lerociclib, a CDK4/6 inhibitor, on pediatric sarcomas in vitro and in 3D bioprinted tumors.
Methods: The effects of lerociclib on viability, proliferation, cell cycle, motility, and stemness were assessed in established sarcoma cell lines, U-2 OS and MG-63, as well as sarcoma patient-derived xenografts (PDXs). 3D printed biotumors of each of the U-2 OS, MG-63, and COA79 cells were utilized to study the effects of lerociclib on tumor growth ex vivo.
Results: CDK 4/6, as well as the intermediaries retinoblastoma protein (Rb) and phosphorylated Rb were identified as targets in the four sarcoma cell lines. Lerociclib treatment induced cell cycle arrest, decreased proliferation, motility, and stemness of sarcoma cells. Treatment with lerociclib decreased sarcoma cell viability in both traditional 2D culture as well as 3D bioprinted microtumors.
Conclusions: Inhibition of CDK 4/6 activity with lerociclib was efficacious in traditional 2D sarcoma cell culture as well as in 3D bioprints. Lerociclib holds promise and warrants further investigation as a novel therapeutic strategy for management of these heterogenous groups of tumors.
Keywords: 3D bioprinting; CDK 4/6 inhibitors; Lerociclib; Pediatric sarcomas.
Copyright © 2023 Elsevier Inc. All rights reserved.
Figures

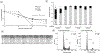

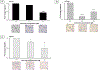

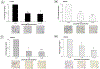
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

