A high-throughput and low-waste viability assay for microbes
- PMID: 37919425
- PMCID: PMC10686820
- DOI: 10.1038/s41564-023-01513-9
A high-throughput and low-waste viability assay for microbes
Abstract
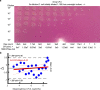
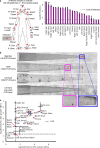
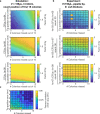
Counting viable cells is a universal practice in microbiology. The colony-forming unit (CFU) assay has remained the gold standard to measure viability across disciplines, but it is time-intensive and resource-consuming. Here we describe the geometric viability assay (GVA) that replicates CFU measurements over 6 orders of magnitude while reducing over 10-fold the time and consumables required. GVA computes a sample's viable cell count on the basis of the distribution of embedded colonies growing inside a pipette tip. GVA is compatible with Gram-positive and Gram-negative planktonic bacteria (Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis), biofilms and fungi (Saccharomyces cerevisiae). Laborious CFU experiments such as checkerboard assays, treatment time-courses and drug screens against slow-growing cells are simplified by GVA. The ease and low cost of GVA evinces that it can replace existing viability assays and enable viability measurements at previously impractical scales.
© 2023. The Author(s).
Conflict of interest statement
C.T.M. and J.M.K. have filed a provisional patent (Provisional US Patent App. 63/334,375, “System And Methods To Measure Cell Viability In High Throughput Via Continuous Geometry,” 26 April, 2022) for the geometric viability assay. The optical imaging system is currently being licensed for commercial use by the University of Colorado Venture Partners. C.T.M. is also a co-founder of Duet BioSystems. A.C. is a founder of Sachi Bio. The remaining authors declare no competing interests.
Figures









Update of
-
High Throughput Viability Assay for Microbiology.bioRxiv [Preprint]. 2023 Jan 4:2023.01.04.522767. doi: 10.1101/2023.01.04.522767. bioRxiv. 2023. Update in: Nat Microbiol. 2023 Dec;8(12):2304-2314. doi: 10.1038/s41564-023-01513-9. PMID: 36712102 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

