Cholesterol induction in CD8+ T cell exhaustion in colorectal cancer via the regulation of endoplasmic reticulum-mitochondria contact sites
- PMID: 37919522
- PMCID: PMC10991466
- DOI: 10.1007/s00262-023-03555-8
Cholesterol induction in CD8+ T cell exhaustion in colorectal cancer via the regulation of endoplasmic reticulum-mitochondria contact sites
Abstract
Background: Hypercholesterolemia is one of the risk factors for colorectal cancer (CRC). Cholesterol can participate in the regulation of human T cell function and affect the occurrence and development of CRC.
Objective: To elucidate the pathogenesis of CRC immune escape mediated by CD8+ T cell exhaustion induced by cholesterol.
Methods: CRC samples (n = 217) and healthy individuals (n = 98) were recruited to analyze the relationship between peripheral blood cholesterol levels and the clinical features of CRC. An animal model of CRC with hypercholesterolemia was established. Intraperitoneal intervention with endoplasmic reticulum stress (ERS) inhibitors in hypercholesterolemic CRC mice was performed. CD69, PD1, TIM-3, and CTLA-4 on CD8+ T cells of spleens from C57BL/6 J mice were detected by flow cytometry. CD8+ T cells were cocultured with MC38 cells (mouse colon cancer cell line). The proliferation, apoptosis, migration and invasive ability of MC38 cells were detected by CCK-8 assay, Annexin-V APC/7-AAD double staining, scratch assay and transwell assay, respectively. Transmission electron microscopy was used to observe the ER structure of CD8+ T cells. Western blotting was used to detect the expression of ERS and mitophagy-related proteins. Mitochondrial function and energy metabolism were measured. Immunoprecipitation was used to detect the interaction of endoplasmic reticulum-mitochondria contact site (ERMC) proteins. Immunofluorescence colocalization was used to detect the expression and intracellular localization of ERMC-related molecules.
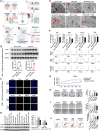
Results: Peripheral blood cholesterol-related indices, including Tc, low density lipoproteins (LDL) and Apo(a), were all increased, and high density lipoprotein (HDL) was decreased in CRCs. The proliferation, migration and invasion abilities of MC38 cells were enhanced, and the proportion of tumor cell apoptosis was decreased in the high cholesterol group. The expression of IL-2 and TNF-α was decreased, while IFN-γ was increased in the high cholesterol group. It indicated high cholesterol could induce exhaustion of CD8+ T cells, leading to CRC immune escape. Hypercholesterolemia damaged the ER structure of CD8+ T cells and increased the expression of ER stress molecules (CHOP and GRP78), lead to CD8+ T cell exhaustion. The expression of mitophagy-related proteins (BNIP3, PINK and Parkin) in exhausted CD8+ T cells increased at high cholesterol levels, causing mitochondrial energy disturbance. High cholesterol enhanced the colocalization of Fis1/Bap31, MFN2/cox4/HSP90B1, VAPB/PTPIP51, VDAC1/IPR3/GRP75 in ERMCs, indicated that high cholesterol promoted the intermolecular interaction between ER and mitochondrial membranes in CD8+ T cells.
Conclusion: High cholesterol regulated the ERS-ERMC-mitophagy axis to induce the exhaustion of CD8+ T cells in CRC.
Keywords: CD8+ T-cell exhaustion; Cholesterol; Colorectal cancer (CRC); ER-mitochondria contact sites (ERMCs); Endoplasmic reticulum stress (ER stress); Mitochondrial energy metabolism.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Figures



References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33 - PubMed
-
- Jun SY, Brown AJ, Chua NK, Yoon JY, Lee JJ, Yang JO, Jang I, Jeon SJ, Choi TI, Kim CH et al (2021) Reduction of squalene epoxidase by cholesterol accumulation accelerates colorectal cancer progression and metastasis. Gastroenterology 160(4):1194-1207.e1128 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

