Real-world treatment patterns of OTX-101 ophthalmic solution, cyclosporine ophthalmic emulsion, and lifitegrast ophthalmic solution in patients with dry eye disease: a retrospective analysis
- PMID: 37919692
- PMCID: PMC10621228
- DOI: 10.1186/s12886-023-03174-y
Real-world treatment patterns of OTX-101 ophthalmic solution, cyclosporine ophthalmic emulsion, and lifitegrast ophthalmic solution in patients with dry eye disease: a retrospective analysis
Abstract
Background: Dry eye disease (DED) is a disorder characterized by loss of tear film homeostasis that causes ocular surface inflammation and damage. The incidence of DED increases with age. Cyclosporine ophthalmic solution 0.09% (CEQUA®; OTX-101), cyclosporine ophthalmic emulsion 0.05% (Restasis®; CsA), and lifitegrast ophthalmic solution 5% (Xiidra®; LFT) are anti-inflammatory agents indicated for DED. This analysis compared treatment patterns in patients with DED receiving OTX-101, CsA, or LFT.
Methods: This real-world, retrospective, longitudinal cohort study utilized Symphony Health Integrated Dataverse claims from July 2019 to June 2021. The dataset included all patients with OTX-101 claims and patients with CsA or LFT claims randomly selected 2:1 to OTX-101. Patients were sorted into 3 cohorts based on index treatment. Index date was that of first treatment claim, and follow-up period was from index date to end of clinical activity or data availability. Time to treatment discontinuation (TTD), probability of discontinuation, and treatment persistence were assessed for OTX-101 vs. CsA, then OTX-101 vs. LFT. Subgroup analysis was performed based on age and prior DED treatment. Kaplan-Meier analysis and log-rank test were used to examine TTD. A logistic model evaluated association between index treatment and discontinuation. Unadjusted and adjusted odds ratios, 95% confidence intervals, and P-values were reported, with statistically significant associations based on P-values < 0.05.
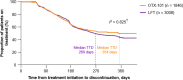
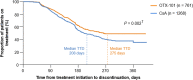
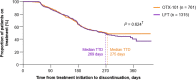
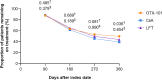
Results: Overall, 7102 patients (OTX-101 n = 1846; CsA n = 2248; LFT n = 3008) were eligible. Median TTD was 354 days for patients receiving OTX-101 vs. 241 days for CsA and 269 days for LFT. Log-rank test indicated TTD was significantly longer for patients on OTX-101 vs. CsA (P = 0.033). Patients on CsA were 35% more likely to discontinue treatment than patients on OTX-101; OTX-101 and LFT groups had similar discontinuation rates. After 360 days, 49.8% of patients receiving OTX-101 remained on treatment vs. 39.4% of patients on CsA (P = 0.036) and 44.0% of patients on LFT (P = 0.854).
Conclusions: Patients receiving OTX-101 remained on treatment significantly longer and were significantly less likely to discontinue treatment than patients on CsA. Older patients remained on OTX-101 significantly longer than CsA. These findings highlight treatment pattern differences in patients with DED receiving these anti-inflammatory agents.
Keywords: Cyclosporine A; Discontinuation; Keratoconjunctivitis sicca; Persistence.
© 2023. The Author(s).
Conflict of interest statement
PK reports consultant fees from Alcon Labs, Aldeyra, Allergan/AbbVie, Azura Pharmaceuticals, Bausch & Lomb, BioTissue, Cambium Pharmaceuticals, Dompé, Imprimis, Mallinckrodt, Oasis Medical, OcuSoft, Oyster Point, RegenerEyes, ScienceBased Health, Silk Technologies, Sun Pharma, Surface Pharmaceuticals, Tarsus Medical, TearClear, Visant Medical, and Vital Tears. VB is Managing Director of Viver Health, LLC, which receives funding from Novartis, BMS, Sun Pharma, and Taiho. BS and BM are employees of Sun Pharmaceutical Industries, Inc. LH, MY, and EZ are employees of the Analysis Group, Inc. AK was an employee of the Analysis Group, Inc. at time of study. CM has received consultant fees from Aerie Pharmaceuticals, Alcon, Bausch & Lomb, Bruder Healthcare, Checked Up, EyePoint, Johnson & Johnson, Kala Pharmaceuticals, Lacriscience, Lenstec, Lumenis, Novartis, Ocular Science, Ocular Therapeutix, Olympic, Quidel, Sun Pharma, TearLab, TissueTech, and Zeiss, and personal fees from Veterinarian Recommended Solutions.
Figures



References
-
- McCann P, Abraham AG, Mukhopadhyay A, Panagiotopoulou K, Chen H, Rittiphairoj T, et al. Prevalence and incidence of dry eye and meibomian gland dysfunction in the United States: a systematic review and meta-analysis. JAMA Ophthalmol. 2022;140(12):1181–1192. doi: 10.1001/jamaophthalmol.2022.4394. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

