Spatiotemporal description of African swine fever virus nucleic acid and antibodies detected in pigs sampled at abattoirs in the greater Kampala metropolitan area, Uganda from May 2021 through June 2022
- PMID: 37919811
- PMCID: PMC10623799
- DOI: 10.1186/s40813-023-00345-7
Spatiotemporal description of African swine fever virus nucleic acid and antibodies detected in pigs sampled at abattoirs in the greater Kampala metropolitan area, Uganda from May 2021 through June 2022
Abstract
Background: African swine fever virus (ASFV) infections in Africa cause hemorrhagic disease in domestic pigs and is maintained by a sylvatic cycle in warthogs. It is endemic in Uganda, leading to significant economic losses. Previous studies performed in rural areas and in Kampala had differing diagnostic results. The purpose of this study was to provide a robust spatial, temporal, and diagnostic summary of pigs slaughtered in the greater Kampala metropolitan area over the course of one year. This study characterized 1208 to 1323 serum, blood, and tissue samples collected from pigs at six abattoirs in the greater Kampala metropolitan area of Uganda monthly from May 2021 through June 2022. Validated and standardized serologic and molecular diagnostics were used.
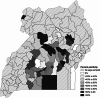
Results: Only 0.15% of pigs had detectable antibodies against ASFV, suggesting low survival rates or pre-clinical diagnosis. Yet, 59.5% of pigs were positive for ASFV DNA. Blood had the lowest detection rate (15.3%) while tonsil and lymph nodes had the highest (38% and 37.5%, respectively), spleen samples (31.5%) were in between. Agreement between sample types was fair to moderate overall. A significant seasonality of ASFV infections emerged with infections found predominately in the dry seasons. Spatial assessments revealed that the greater Kampala metropolitan area abattoirs have a catchment area that overlaps with Uganda's most pig dense regions.
Conclusions: Pigs at greater Kampala metropolitan area abattoirs can be sentinels for acute disease throughout the pig dense region of Uganda, particularly in the dry seasons. The high prevalence detected suggests that pigs are sold in response to local reports of ASFV infections (panic sales). Serological surveillance is not useful, as very few pigs seroconverted in this study prior to slaughter. In contrast, tissue samples of pigs can be used to detect disease using qPCR methods.
Keywords: African swine Fever; ELISA; Uganda; qPCR.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Haresnape JM, Wilkinson PJ. A study of African swine Fever virus infected ticks (Ornithodoros moubata) collected from three villages in the ASF enzootic area of Malawi following an outbreak of the Disease in domestic pigs. Epidemiol Infect. 1989;102(3):507–22. doi: 10.1017/S0950268800030223. - DOI - PMC - PubMed
Grants and funding
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
- HDTRA1-20-1-0007/Department of the Defense, Defense Threat Reduction Agency
LinkOut - more resources
Full Text Sources

