Flavone-based dual PARP-Tubulin inhibitor manifesting efficacy against endometrial cancer
- PMID: 37919954
- PMCID: PMC10627047
- DOI: 10.1080/14756366.2023.2276665
Flavone-based dual PARP-Tubulin inhibitor manifesting efficacy against endometrial cancer
Abstract
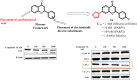
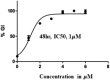
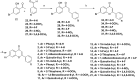
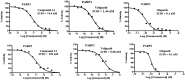
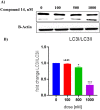
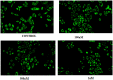
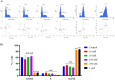
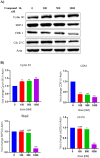
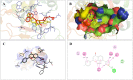
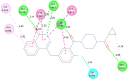
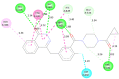
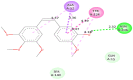
Structural tailoring of the flavone framework (position 7) via organopalladium-catalyzed C-C bond formation was attempted in this study. The impact of substituents with varied electronic effects (phenyl ring, position 2 of the benzopyran scaffold) on the antitumor properties was also assessed. Resultantly, the efforts yielded a furyl arm bearing benzopyran possessing a 4-fluoro phenyl ring (position 2) (14) that manifested a magnificent antitumor profile against the Ishikawa cell lines mediated through dual inhibition of PARP and tubulin [(IC50 (PARP1) = 74 nM, IC50 (PARP2) = 109 nM) and tubulin (IC50 = 1.4 µM)]. Further investigations confirmed the ability of 14 to induce apoptosis as well as autophagy and cause cell cycle arrest at the G2/M phase. Overall, the outcome of the study culminated in a tractable dual PARP-tubulin inhibitor endowed with an impressive activity profile against endometrial cancer.
Keywords: Flavone; PARP; benzopyran; endometrial cancer; inhibitor; tubulin.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









Similar articles
-
Discovery of a Dual Tubulin and Poly(ADP-Ribose) Polymerase-1 Inhibitor by Structure-Based Pharmacophore Modeling, Virtual Screening, Molecular Docking, and Biological Evaluation.J Med Chem. 2021 Nov 11;64(21):15702-15715. doi: 10.1021/acs.jmedchem.1c00932. Epub 2021 Oct 21. J Med Chem. 2021. PMID: 34670362
-
Identification of new 3-phenyl-1H-indole-2-carbohydrazide derivatives and their structure-activity relationships as potent tubulin inhibitors and anticancer agents: A combined in silico, in vitro and synthetic study.Bioorg Chem. 2021 May;110:104795. doi: 10.1016/j.bioorg.2021.104795. Epub 2021 Mar 4. Bioorg Chem. 2021. PMID: 33730670
-
Design, synthesis, and biological evaluation of novel diphenylamine derivatives as tubulin polymerization inhibitors targeting the colchicine binding site.Eur J Med Chem. 2022 Jul 5;237:114372. doi: 10.1016/j.ejmech.2022.114372. Epub 2022 Apr 16. Eur J Med Chem. 2022. PMID: 35447432
-
Discovery of novel tubulin/HDAC dual-targeting inhibitors with strong antitumor and antiangiogenic potency.Eur J Med Chem. 2021 Dec 5;225:113790. doi: 10.1016/j.ejmech.2021.113790. Epub 2021 Aug 19. Eur J Med Chem. 2021. PMID: 34454126
-
Design and discovery of new antiproliferative 1,2,4-triazin-3(2H)-ones as tubulin polymerization inhibitors targeting colchicine binding site.Bioorg Chem. 2021 Jul;112:104965. doi: 10.1016/j.bioorg.2021.104965. Epub 2021 May 5. Bioorg Chem. 2021. PMID: 34020238
References
-
- Nagy JD, Armbruster D.. Evolution of uncontrolled proliferation and the angiogenic switch in cancer. Math Biosci Eng. 2012;9(4):843–876. - PubMed
-
- Kuo CN, Liao YM, Kuo LN, Tsai HJ, Chang WC, Yen Y.. Cancers in Taiwan: practical insight from epidemiology, treatments, biomarkers, and cost. J Formos Med Assoc. 2020; 119(12):1731–1741. - PubMed
-
- Doll R, Peto R.. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1192–1308. - PubMed
-
- Trichopoulos D, Li FP, Hunter DJ.. What causes cancer? Sci Am. 1996;275(3):80–87. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
