Surface engineering on a microporous metal-organic framework to boost ethane/ethylene separation under humid conditions
- PMID: 37920341
- PMCID: PMC10619615
- DOI: 10.1039/d3sc04119k
Surface engineering on a microporous metal-organic framework to boost ethane/ethylene separation under humid conditions
Abstract
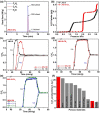
Recently, examples of metal-organic frameworks (MOFs) have been identified displaying ethane (C2H6) over ethylene (C2H4) adsorption selectivity. However, it remains a challenge to construct MOFs with both large C2H6 adsorption capacity and high C2H6/C2H4 adsorption selectivity, especially under humid conditions. Herein, we reported two isoreticular MOF-5 analogues (JNU-6 and JNU-6-CH3) and their potential applications in one-step separation of C2H4 from C2H6/C2H4 mixtures. The introduction of CH3 groups not only reduces the pore size from 5.4 Å in JNU-6 to 4.1 Å in JNU-6-CH3 but also renders an increased electron density on the pyrazolate N atoms of the organic linker. JNU-6-CH3 retains its framework integrity even after being immersed in water for six months. More importantly, it exhibits large C2H6 adsorption capacity (4.63 mmol g-1) and high C2H6/C2H4 adsorption selectivity (1.67) due to the optimized pore size and surface function. Breakthrough experiments on JNU-6-CH3 demonstrate that C2H4 can be directly separated from C2H6/C2H4 (50/50, v/v) mixtures, affording benchmark productivity of 22.06 and 18.71 L kg-1 of high-purity C2H4 (≥99.95%) under dry and humid conditions, respectively.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources

