Vascular smooth muscle cell mechanotransduction through serum and glucocorticoid inducible kinase-1 promotes interleukin-6 production and macrophage accumulation in murine hypertension
- PMID: 37920479
- PMCID: PMC10618507
- DOI: 10.1016/j.jvssci.2023.100124
Vascular smooth muscle cell mechanotransduction through serum and glucocorticoid inducible kinase-1 promotes interleukin-6 production and macrophage accumulation in murine hypertension
Abstract
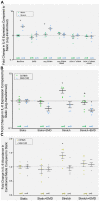
Objective: The objective of this investigation was to demonstrate that in vivo induction of hypertension (HTN) and in vitro cyclic stretch of aortic vascular smooth muscle cells (VSMCs) can cause serum and glucocorticoid-inducible kinase (SGK-1)-dependent production of cytokines to promote macrophage accumulation that may promote vascular pathology.
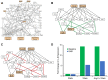
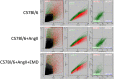
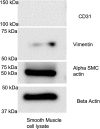
Methods: HTN was induced in C57Bl/6 mice with angiotensin II infusion (1.46 mg/kg/day × 21 days) with or without systemic infusion of EMD638683 (2.5 mg/kg/day × 21 days), a selective SGK-1 inhibitor. Systolic blood pressure was recorded. Abdominal aortas were harvested to quantify SGK-1 activity (pSGK-1/SGK-1) by immunoblot. Flow cytometry quantified the abundance of CD11b+/F480+ cells (macrophages). Plasma interleukin (IL)-6 and monocyte chemoattractant protein-1 (MCP-1) was assessed by enzyme-linked immunosorbent assay. Aortic VSMCs from wild-type mice were subjected to 12% biaxial cyclic stretch (Stretch) for 3 or 12 hours with or without EMD638683 (10 μM) and with or without SGK-1 small interfering RNA with subsequent quantitative polymerase chain reaction for IL-6 and MCP-1 expression. IL-6 and MCP-1 in culture media were analyzed by enzyme-linked immunosorbent assay. Aortic VSMCs from SGK-1flox+/+ mice were transfected with Cre-Adenovirus to knockdown SGK-1 (SGK-1KD VSMCs) and underwent parallel tension experimentation. Computational modeling was used to simulate VSMC signaling. Statistical analysis included analysis of variance with significance at a P value of <.05.
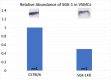
Results: SGK-1 activity, abundance of CD11b+/F4-80+ cells, and plasma IL-6 were increased in the abdominal aorta of mice with HTN and significantly reduced by treatment with EMD638683. This outcome mirrored the increased abundance of IL-6 in media from Stretch C57Bl/6 VSMCs and attenuation of the effect with EMD638683 or SGK-1 small interfering RNA. C57Bl/6 VSMCs also responded to Stretch with increased MCP-1 expression and secretion into the culture media. Further supporting the integral role of mechanical signaling through SGK-1, target gene expression and cytokine secretion was unchanged in SGK-1KD VSMCs with Stretch, and computer modeling confirmed SGK-1 as an intersecting node of signaling owing to mechanical strain and angiotensin II.
Conclusions: Mechanical activation of SGK-1 in aortic VSMCs can promote inflammatory signaling and increased macrophage abundance, therefore this kinase warrants further exploration as a pharmacotherapeutic target to abrogate hypertensive vascular pathology.
Keywords: Hypertension; Interleukin-6; Serum and glucocorticoid-inducible kinase-1 (SGK-1).
Conflict of interest statement
J.M.R. is a surgical proctor for CVRx, outside the scope of the submitted work.
Figures



References
-
- Haga J.H., Li Y.S., Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–960. - PubMed
-
- Anwar M.A., Shalhoub J., Lim C.S., Gohel M.S., Davies A.H. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 2012;49:463–478. - PubMed
-
- Cheng J., Wang Y., Ma Y., et al. The mechanical stress-activated serum-, glucocorticoid-regulated kinase 1 contributes to neointima formation in vein grafts. Circ Res. 2010;107:1265–1274. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

