The impact of data quality monitoring of a multicenter prospective registry of cardiac implantable electronic devices
- PMID: 37920872
- PMCID: PMC10618759
- DOI: 10.1016/j.mex.2023.102454
The impact of data quality monitoring of a multicenter prospective registry of cardiac implantable electronic devices
Abstract
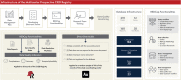
Data quality monitoring plays a crucial role in multicenter prospective registries. By maintaining high data accuracy, completeness, and consistency, researchers can improve the overall quality and reliability of the registry data, enabling meaningful conclusions and supporting evidence-based decisions. The purpose of the present study was to evaluate data quality metrics (completeness, accuracy, and temporal plausibility) of a Multicenter Registry of Cardiac Implantable Electronic Devices (CIEDs) and to perform a direct data audit of a random sample of records to assess the agreement levels with the source documents. The CIED Registry was a prospective, multicenter, real-world observational study carried out from January 2020 to December 2022 in five designated centers across Sao Paulo, Brazil. We assessed the data quality of the CIED Registry by using two distinct approaches:•Dynamic data monitoring using features of the REDCap (Research Electronic Data Capture) software, including data reports and data quality rules•Direct data audit in which information from a random sample of 10 % of cases from the coordinating center was compared with original source documents Our findings suggest that the methodological approach applied to the CIED Registry resulted in high data completeness, accuracy, temporal plausibility, and excellent agreement levels with the source documents.
Keywords: Artificial; Data audit; Data management; Data quality; Data quality monitoring of multicenter registries; Database; Pacemaker; REDCap; Registries.
© 2023 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Gliklich R.E., Leavy M.B. Assessing real-world data quality: the application of patient registry quality criteria to real-world data and real-world evidence. Ther Innov Regul Sci. 2020;54(2):303–307. - PubMed
-
- Malenka D.J., Bhatt D.L., Bradley S.M., Shahian D.M., Draoui J., Segawa C.A., et al. The national cardiovascular data registry data quality program 2020: JACc state-of-the-art review. J. Am. Coll. Cardiol. 2022;79(17):1704–1712. - PubMed
-
- Faxon D.P., Burgess A. Cardiovascular registries: too much of good thing? Circ. Cardiovasc. Interv. 2016;9(4) - PubMed
-
- Masoudi F.M., Oetgen W.J. The NCDR ICD registry: a foundation for quality improvement. J. Am. Coll. Cardiol. 2017;70(13):1673–1674. - PubMed
LinkOut - more resources
Full Text Sources