Protocol of the IntenSify-Trial: An open-label phase I trial of the CYP3A inhibitor cobicistat and the cytostatics gemcitabine and nab-paclitaxel in patients with advanced stage or metastatic pancreatic ductal adenocarcinoma to evaluate the combination's pharmacokinetics, safety, and efficacy
- PMID: 37920921
- PMCID: PMC10719473
- DOI: 10.1111/cts.13661
Protocol of the IntenSify-Trial: An open-label phase I trial of the CYP3A inhibitor cobicistat and the cytostatics gemcitabine and nab-paclitaxel in patients with advanced stage or metastatic pancreatic ductal adenocarcinoma to evaluate the combination's pharmacokinetics, safety, and efficacy
Abstract
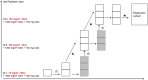
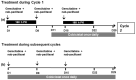
Expression of CYP3A5 protein is a basal and acquired resistance mechanism of pancreatic ductal adenocarcinoma cells conferring protection against the CYP3A and CYP2C8 substrate paclitaxel through metabolic degradation. Inhibition of CYP3A isozymes restores the cells sensitivity to paclitaxel. The combination of gemcitabine and nab-paclitaxel is an established regimen for the treatment of metastasized or locally advanced inoperable pancreatic cancer. Cobicistat is a CYP3A inhibitor developed for the pharmacoenhancement of protease inhibitors. The addition of cobicistat to gemcitabine and nab-paclitaxel may increase the antitumor effect. We will conduct a phase I dose escalation trial with a classical 3 + 3 design to investigate the safety, tolerability, and pharmacokinetics (PKs) of gemcitabine, nab-paclitaxel, and cobicistat. Although the doses of gemcitabine (1000 mg/m2 ) and cobicistat (150 mg) are fixed, three dose levels of nab-paclitaxel (75, 100, and 125 mg/m2 ) will be explored to account for a potential PK drug interaction. After the dose escalation phase, we will set the recommended dose for expansion (RDE) and treat up to nine patients in an expansion part of the trial. The trial is registered under the following identifiers EudraCT-Nr. 2019-001439-29, drks.de: DRKS00029409, and ct.gov: NCT05494866. Overcoming resistance to paclitaxel by CYP3A5 inhibition may lead to an increased efficacy of the gemcitabine and nab-paclitaxel regimen. Safety, efficacy, PK, and RDE data need to be acquired before investigating this combination in a large-scale clinical study.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.R.S. and A.T. are holding the patient WO/2016/045799, NOVEL METHODS FOR SUB‐TYPING AND TREATING CANCER. A.S. receives honoraria for advisory boards or speaker's bureau from Agilent, Aignostics, Amgen, Astra Zeneca, Bayer, BMS, Eli Lilly, Illumina, Incyte, Janssen, MSD, Novartis, Pfizer, Qlucore, Roche, Seagen, Takeda, Thermo Fisher and grants from Bayer, BMS, Chugai, Incyte, all outside the submitted work. J.T.S. receives honoraria as consultant or for continuing medical education presentations from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Immunocore, MSD Sharp Dohme, Novartis, Roche/Genentech, and Servier. His institution receives research funding from Abalos Therapeutics, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eisbach Bio, and Roche/Genentech; he holds ownership and serves on the Board of Directors of Pharma15, all outside the submitted work. T.S. receives honoraria as consultant or for continuing medical education presentations from BMS, Cantargia, Mirati, Olympus America, Inc., Pierre Fabre, Scandion, Servier and Research support from Lilly, all outside the submitted work. All other authors declared no competing interests for this work.
Figures
References
-
- NCI SEERS Database . Cancer stat facts: pancreatic cancer. Accessed June 15, 2023. https://seer.cancer.gov/statfacts/html/pancreas.html. Last
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J Clin Oncol. 2007;25:1960‐1966. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817‐1825. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

