Real-World Persistence of Successive Biologics in Patients With Inflammatory Bowel Disease: Findings From ROTARY
- PMID: 37921344
- PMCID: PMC11447059
- DOI: 10.1093/ibd/izad245
Real-World Persistence of Successive Biologics in Patients With Inflammatory Bowel Disease: Findings From ROTARY
Abstract
Background: Patients with inflammatory bowel disease (IBD) may receive multiple successive biologic treatments in clinical practice; however, data are limited on the comparative effectiveness of biologics and the impact of treatment sequence on outcomes.
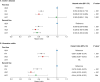
Methods: The ROTARY (Real wOrld ouTcomes Across tReatment sequences in inflammatorY bowel disease patients) study was a retrospective, observational cohort study conducted using data from the Optum Clinical Database between January 1, 2012, and February 29, 2020. Adult patients with Crohn's disease (CD) or ulcerative colitis (UC) who received 2 biologics successively were included. Biologic treatment sequences were analyzed descriptively. Cox proportional hazards models, adjusted for baseline demographics and clinical characteristics, were used to estimate the hazard ratio of switching or discontinuation for each first- and second-line biologic compared with first- and second-line adalimumab, respectively.
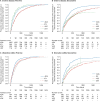
Results: In total, 4648 patients with IBD (CD, n = 3008; UC, n = 1640) were identified. Most patients received tumor necrosis factor α antagonist (anti-TNFα) treatment followed by another anti-TNFα treatment or vedolizumab. Vedolizumab and infliximab had 39.4% and 34.6% lower rates of switching or discontinuation than adalimumab, respectively, as first-line biologics in patients with CD and 30.8% and 34.3% lower rates as first-line biologics in patients with UC, respectively. Vedolizumab, infliximab, and ustekinumab had 47.2%, 40.0%, and 43.5% lower rates of switching or discontinuation than adalimumab, respectively, as second-line biologics in CD and 56.5%, 43.0%, and 45.6% lower rates as second-line biologics in patients with UC, respectively.
Conclusions: Although anti-TNFα treatments were most commonly prescribed, the adjusted rates of discontinuation for adalimumab as both a first- and second-line biologic were higher than for vedolizumab, infliximab, or ustekinumab.
Keywords: Crohn’s disease; biologics; inflammatory bowel disease; sequencing; ulcerative colitis.
Plain language summary
Patients with inflammatory bowel disease are commonly treated with different sequences of biologics. This study shows that patients who receive adalimumab as their first or second biologic treatment either stop or switch to another biologic at a greater rate than those who are treated with vedolizumab, infliximab, and ustekinumab.
© 2023 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
N.K.C. has served as a consultant for NeuroLogica and Takeda Pharmaceuticals USA, Inc. and received speaker's fees from Bristol Myers Squibb. B.C. and T.B. are employees of Optum. S.G. was an employee of Takeda Pharmaceuticals USA, Inc at the time of the study and had stock or stock options. N.C. and T.F. are employees of Takeda Pharmaceuticals USA, Inc and have stock or stock options. K.U. was an employee of University of Illinois, Chicago, IL, USA, supported by a Takeda Pharmaceuticals USA, Inc fellowship at the time of the study. D.T.R. has received grant support from Takeda Pharmaceuticals USA, Inc. and served as a consultant for AbbVie, AltruBio, Arena Pharmaceuticals, Bristol Myers Squibb, Genentech/Roche, Gilead Sciences, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Pfizer, Prometheus Biosciences, Takeda, and TechLab Inc.
Figures

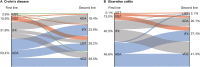
References
-
- Kornbluth A, Sachar DB.. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105(3):501-23; quiz 524. - PubMed
-
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ.. Ulcerative colitis. Lancet. 2012;380(9853):1606-1619. - PubMed
-
- Roda G, Chien Ng S, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers. 2020;6(1):22. - PubMed
-
- Sulz MC, Burri E, Michetti P, Rogler G, Peyrin-Biroulet L, Seibold F; on behalf of the Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Treatment algorithms for Crohn’s disease. Digestion. 2020;101(Suppl 1):43-57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

