Subcutaneous epcoritamab monotherapy in Japanese adults with relapsed/refractory diffuse large B-cell lymphoma
- PMID: 37921363
- PMCID: PMC10728012
- DOI: 10.1111/cas.15996
Subcutaneous epcoritamab monotherapy in Japanese adults with relapsed/refractory diffuse large B-cell lymphoma
Abstract
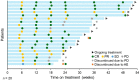
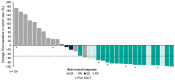
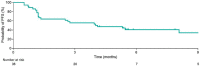
Epcoritamab is a subcutaneously administered CD3xCD20 bispecific Ab that showed deep, durable responses with a manageable safety profile in patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) in the global multicenter pivotal phase II trial EPCORE NHL-1. Here, we present results from the similar EPCORE NHL-3 phase I/II trial evaluating epcoritamab monotherapy in Japanese patients with R/R CD20+ B-cell non-Hodgkin's lymphoma previously treated with two or more lines of therapy. Epcoritamab was dosed subcutaneously in 28-day cycles; once weekly during cycles 1-3, every 2 weeks during cycles 4-9, and every 4 weeks from cycle 10 until disease progression or unacceptable toxicity. Step-up dosing and cytokine release syndrome (CRS) prophylaxis were used during treatment cycle 1. As of January 31, 2022, 36 patients received treatment with 48 mg epcoritamab monotherapy. At a median follow-up of 8.4 months, overall response and complete response rates by independent review committee were 55.6% and 44.4%, respectively. The median duration of response, duration of complete response, and overall survival were not reached at the time of data cut-off. The most common treatment-emergent adverse events of any grade were CRS (83.3%), injection-site reactions (69.4%), infections (44.4%), neutropenia (38.9%), hypokalemia (27.8%), and decreased lymphocyte count (25.0%). Cytokine release syndrome occurrence was predictable; events were primarily low grade (grade 1-2), all resolved, and none led to treatment discontinuation. These encouraging results are consistent with previous findings and support the ongoing clinical evaluation of epcoritamab for the treatment of R/R DLBCL, including in earlier treatment lines.
Keywords: B-cell lymphoma; CD20 antigen; CD3 antigen; bispecific antibody; diffuse large B-cell lymphoma.
© 2023 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Koji Izutsu has received honoraria/fees from Janssen and Ono Pharmaceutical and research funding from AbbVie, Incyte Biosciences, Bristol Myers Squibb, Novartis, Janssen, Yakult, Daiichi Sankyo, Chugai, BeiGene, and Genmab. Hirokazu Nagai has received honoraria/fees from Chugai, Janssen, Meiji Seika, and Ono Pharmaceutical and scholarship endowments/academic research funding from Chugai, AstraZeneca, Janssen, Genmab, AbbVie, Daiichi Sankyo, BeiGene, Eli Lilly, and Kyowa Kirin. Yuko Mishima has received honoraria/fees from Chugai and Roche and scholarship endowments/academic research funding from Takeda, Eisai, and Bristol Myers Squibb. Youko Suehiro has received research funding from Chugai, Genmab, Incyte, Amgen, Otsuka, AbbVie, Kyowa Kirin, and All Japan Pharma. Kazuhito Yamamoto has received honoraria/fees from AbbVie, Chugai, Eisai, HUYA, IQVIA, Janssen, Meiji Seika Pharma, Micron, Daiichi Sankyo, and Takeda and research funding from Genmab, IQVIA, and Yakult. Kenichi Ishizawa has received honoraria/fees from Bristol Myers Squibb, Chugai, Eisai, and Novartis and research funding from Pfizer, SymBio, AbbVie, Chugai, and Zenyaku. Takayuki Ikezoe has received honoraria/fees from Chugai, Asahi Kasei, and Nippon Shinyaku, research funding from Asahi Kasei, and scholarship endowments/academic research funding from Asahi Kasei, Novartis, Alexion, Janssen, Dainippon Sumitomo, Nippon Shinyaku, Takeda, AbbVie, Incyte, Otsuka, and GSK. Momoko Nishikori has received honoraria/fees from Chugai and Janssen and research funding from SymBio. Minh Dinh is an employee of AbbVie. David Soong, Hidehisa Noguchi, Jeppe Klint Buchbjerg, and Elena Favaro are employees of Genmab. Noriko Fukuhara has received honoraria/fees from Bristol Myers Squibb, Chugai, HUYA, and SymBio and research funding from AbbVie, Bayer, Celgene, Chordia, Chugai, Genmab, Incyte, Kyowa Kirin, Loxo Oncology, and Takeda. The other authors have no conflict of interest. The study was funded by Genmab A/S and AbbVie. Epcoritamab was provided by Genmab A/S and AbbVie. Genmab A/S and AbbVie collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Figures


References
-
- B‐Cell Non‐Hodgkin's Lymphoma (NHL) . Opportunity Analysis and Forecasts to 2027. 2019. GlobalData, Accessed September 6, 2023. Available at: https://www.globaldata.com/store/report/b‐cell‐non‐hodgkins‐lymphoma‐nhl...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

