Safety and immunogenicity of three dose levels of an investigational, highly purified Vero cell rabies vaccine: A randomized, controlled, observer-blinded, Phase II study with a simulated post-exposure regimen in healthy adults
- PMID: 37921410
- PMCID: PMC10627063
- DOI: 10.1080/21645515.2023.2275453
Safety and immunogenicity of three dose levels of an investigational, highly purified Vero cell rabies vaccine: A randomized, controlled, observer-blinded, Phase II study with a simulated post-exposure regimen in healthy adults
Abstract
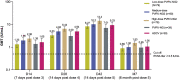
A serum-free, highly purified rabies vaccine produced in Vero cells is under development. The initial formulation, PVRV-NG, was evaluated in five Phase II studies and subsequently reformulated (PVRV-NG2). This multicenter, observer-blinded Phase II study investigated the safety and immune response of three different doses (antigen content) of PVRV-NG2 versus a licensed human diploid cell rabies vaccine (HDCV; Imovax rabies®). Healthy adults (N = 320) were randomized to receive PVRV-NG2 (low, medium, or high dose), PVRV-NG, or HDCV (2:2:2:1:1 ratio), according to a five-dose Essen simulated post-exposure regimen (Days [D] 0, 3, 7, 14, and 28). All participants received human rabies immunoglobulin intramuscularly on D0. Immunogenicity was assessed at D0, 14, 28, 42, and 6 months after the final injection using the rapid fluorescent focus inhibition test. Seroconversion rates were calculated as the percentage of participants achieving rabies virus neutralizing antibody titers ≥0.5 IU/mL. All analyses were descriptive. At each timepoint, geometric mean titers (GMTs) increased with antigen content (measured using an enzyme-linked immunosorbent assay). High-dose PVRV-NG2 GMTs were the highest at all timepoints, medium-dose PVRV-NG2 GMTs were similar to those with HDCV, and low-dose PVRV-NG2 GMTs were similar to PVRV-NG. The safety profile of PVRV-NG2 was comparable to PVRV-NG; however, fewer injection site reactions were reported with PVRV-NG2 or PVRV-NG (range 36.7-47.5%) than with HDCV (61.5%). This study demonstrated a dose-effect of antigen content at all timepoints. As post-exposure prophylaxis, the safety and immunogenicity profiles of the high-dose PVRV-NG2 group compared favorably with HDCV. Clinicaltrials.gov number: NCT03145766.
Keywords: HDCV; Immunogenicity; Vero cell rabies vaccine – serum free; post-exposure prophylaxis; rabies; safety.
Conflict of interest statement
JS and BE declare they have no conflicts of interest. SP, FGM, ACPP, AM, CP, and AMM are employees of Sanofi.
Figures


Similar articles
-
Immunogenicity and Safety of a Purified Vero Rabies Vaccine-Serum Free, Compared With 2 Licensed Vaccines, in a Simulated Rabies Post-Exposure Regimen in Healthy Adults in France: A Randomized, Controlled, Phase 3 Trial.Clin Infect Dis. 2024 Jun 14;78(6):1748-1756. doi: 10.1093/cid/ciae137. Clin Infect Dis. 2024. PMID: 38478634 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a serum-free purified Vero rabies vaccine in comparison with the rabies human diploid cell vaccine (HDCV; Imovax® Rabies) administered in a simulated rabies post-exposure regimen in healthy adults.Vaccine. 2024 Apr 11;42(10):2553-2559. doi: 10.1016/j.vaccine.2023.11.052. Epub 2023 Dec 16. Vaccine. 2024. PMID: 38105138
-
Non-inferiority study of purified Vero rabies vaccine - serum free in three-dose and two-dose pre-exposure prophylaxis regimens in comparison with licensed rabies vaccines.Clin Infect Dis. 2024 Nov 26:ciae581. doi: 10.1093/cid/ciae581. Online ahead of print. Clin Infect Dis. 2024. PMID: 39587931
-
Immunogenicity and safety of human diploid cell vaccine (HDCV) vs. purified Vero cell vaccine (PVRV) vs. purified chick embryo cell vaccine (PCECV) used in post-exposure prophylaxis: a systematic review and meta-analysis.Hum Vaccin Immunother. 2022 Dec 31;18(1):2027714. doi: 10.1080/21645515.2022.2027714. Epub 2022 Feb 22. Hum Vaccin Immunother. 2022. PMID: 35192787 Free PMC article.
-
Purified Vero Cell Rabies Vaccine (PVRV, Verorab®): A Systematic Review of Intradermal Use Between 1985 and 2019.Trop Med Infect Dis. 2020 Mar 7;5(1):40. doi: 10.3390/tropicalmed5010040. Trop Med Infect Dis. 2020. PMID: 32156005 Free PMC article. Review.
Cited by
-
Infection and Prevention of Rabies Viruses.Microorganisms. 2025 Feb 9;13(2):380. doi: 10.3390/microorganisms13020380. Microorganisms. 2025. PMID: 40005749 Free PMC article. Review.
-
Immunogenicity and Safety of a Purified Vero Rabies Vaccine-Serum Free, Compared With 2 Licensed Vaccines, in a Simulated Rabies Post-Exposure Regimen in Healthy Adults in France: A Randomized, Controlled, Phase 3 Trial.Clin Infect Dis. 2024 Jun 14;78(6):1748-1756. doi: 10.1093/cid/ciae137. Clin Infect Dis. 2024. PMID: 38478634 Free PMC article. Clinical Trial.
-
Randomized Controlled Trial of the Immunogenicity and Safety of a Serum-Free Purified Vero Rabies Vaccine (PVRV-NG2) Using a Simulated Postexposure Zagreb Regimen With Human Rabies Immunoglobulin in Adults in Thailand.Open Forum Infect Dis. 2024 Oct 25;11(11):ofae633. doi: 10.1093/ofid/ofae633. eCollection 2024 Nov. Open Forum Infect Dis. 2024. PMID: 39544495 Free PMC article.
References
-
- World Health Organization . Rabies epidemiology and burden of disease; 2022. [accessed 2023 Sept 1]. https://www.who.int/teams/control-of-neglected-tropical-diseases/rabies/....
-
- World Health Organization . Rabies fact sheet; 2021. [accessed 2023 Sept 1]. https://www.who.int/news-room/fact-sheets/detail/rabies.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
