Putative novel hydrogen- and iron-oxidizing sheath-producing Zetaproteobacteria thrive at the Fåvne deep-sea hydrothermal vent field
- PMID: 37921472
- PMCID: PMC10734525
- DOI: 10.1128/msystems.00543-23
Putative novel hydrogen- and iron-oxidizing sheath-producing Zetaproteobacteria thrive at the Fåvne deep-sea hydrothermal vent field
Abstract
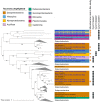
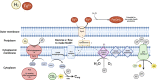
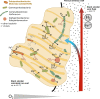
Knowledge on microbial iron oxidation is important for understanding the cycling of iron, carbon, nitrogen, nutrients, and metals. The current study yields important insights into the niche sharing, diversification, and Fe(III) oxyhydroxide morphology of Ghiorsea, an iron- and hydrogen-oxidizing Zetaproteobacteria representative belonging to Zetaproteobacteria operational taxonomic unit 9. The study proposes that Ghiorsea exhibits a more extensive morphology of Fe(III) oxyhydroxide than previously observed. Overall, the results increase our knowledge on potential drivers of Zetaproteobacteria diversity in iron microbial mats and can eventually be used to develop strategies for the cultivation of sheath-forming Zetaproteobacteria.
Keywords: Zetaproteobacteria; genome-resolved metagenomics; hydrogen oxidation; hydrothermal vents; iron oxidation; microbial mats.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Microbial iron mats at the Mid-Atlantic Ridge and evidence that Zetaproteobacteria may be restricted to iron-oxidizing marine systems.PLoS One. 2015 Mar 11;10(3):e0119284. doi: 10.1371/journal.pone.0119284. eCollection 2015. PLoS One. 2015. PMID: 25760332 Free PMC article.
-
Hidden diversity revealed by genome-resolved metagenomics of iron-oxidizing microbial mats from Lō'ihi Seamount, Hawai'i.ISME J. 2017 Aug;11(8):1900-1914. doi: 10.1038/ismej.2017.40. Epub 2017 Apr 14. ISME J. 2017. PMID: 28362721 Free PMC article.
-
The Fe(II)-oxidizing Zetaproteobacteria: historical, ecological and genomic perspectives.FEMS Microbiol Ecol. 2019 Apr 1;95(4):fiz015. doi: 10.1093/femsec/fiz015. FEMS Microbiol Ecol. 2019. PMID: 30715272 Free PMC article. Review.
-
Comparative Analysis of Microbial Communities in Iron-Dominated Flocculent Mats in Deep-Sea Hydrothermal Environments.Appl Environ Microbiol. 2016 Sep 16;82(19):5741-55. doi: 10.1128/AEM.01151-16. Print 2016 Oct 1. Appl Environ Microbiol. 2016. PMID: 27422841 Free PMC article.
-
The microbiomes of deep-sea hydrothermal vents: distributed globally, shaped locally.Nat Rev Microbiol. 2019 May;17(5):271-283. doi: 10.1038/s41579-019-0160-2. Nat Rev Microbiol. 2019. PMID: 30867583 Review.
Cited by
-
Microbial community response to hydrocarbon exposure in iron oxide mats: an environmental study.Front Microbiol. 2024 May 10;15:1388973. doi: 10.3389/fmicb.2024.1388973. eCollection 2024. Front Microbiol. 2024. PMID: 38800754 Free PMC article.
-
Oxidation of sulfur, hydrogen, and iron by metabolically versatile Hydrogenovibrio from deep sea hydrothermal vents.ISME J. 2024 Jan 8;18(1):wrae173. doi: 10.1093/ismejo/wrae173. ISME J. 2024. PMID: 39276367 Free PMC article.
References
-
- Sowers TD, Harrington JM, Polizzotto ML, Duckworth OW. 2017. Sorption of arsenic to biogenic iron (oxyhydr)oxides produced in circumneutral environments. Geochim Cosmochim Acta 198:194–207. doi:10.1016/j.gca.2016.10.049 - DOI
-
- Koschinsky A, Winkler A, Fritsche U. 2003. Importance of different types of marine particles for the scavenging of heavy metals in the deep-sea bottom water. Appl Geochem 18:693–710. doi:10.1016/S0883-2927(02)00161-0 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

