Sequencing and culture-based characterization of the vaginal and uterine microbiota in beef cattle that became pregnant or remained open following artificial insemination
- PMID: 37921486
- PMCID: PMC10714821
- DOI: 10.1128/spectrum.02732-23
Sequencing and culture-based characterization of the vaginal and uterine microbiota in beef cattle that became pregnant or remained open following artificial insemination
Abstract
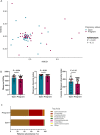
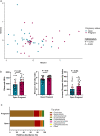
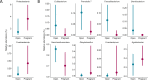
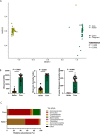
Emerging evidence suggests that microbiome-targeted approaches may provide a novel opportunity to reduce the incidence of reproductive failures in cattle. To develop such microbiome-based strategies, one of the first logical steps is to identify reproductive microbiome features related to fertility and to isolate the fertility-associated microbial species for developing a future bacterial consortium that could be administered before breeding to enhance pregnancy outcomes. Here, we characterized the vaginal and uterine microbiota in beef cattle that became pregnant or remained open via artificial insemination and identified microbiota features associated with fertility. We compared similarities between vaginal and uterine microbiota and between heifers and cows. Using culturing, we provided new insights into the culturable fraction of the vaginal and uterine microbiota and their antimicrobial resistance. Overall, our findings will serve as an important basis for future research aimed at harnessing the vaginal and uterine microbiome for improved cattle fertility.
Keywords: 16S rRNA gene sequencing; antimicrobial resistance; artificial insemination; bovine; culturing; pregnancy; uterine microbiota; vaginal microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




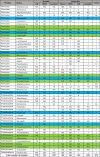
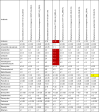

Similar articles
-
Vaginal bacterial community composition and concentrations of estradiol at the time of artificial insemination in Brangus heifers.J Anim Sci. 2020 Jun 1;98(6):skaa178. doi: 10.1093/jas/skaa178. J Anim Sci. 2020. PMID: 32515480 Free PMC article.
-
Uterine and vaginal bacterial community diversity prior to artificial insemination between pregnant and nonpregnant postpartum cows1.J Anim Sci. 2019 Oct 3;97(10):4298-4304. doi: 10.1093/jas/skz210. J Anim Sci. 2019. PMID: 31250893 Free PMC article.
-
Metataxonomic Analysis of the Uterine Microbiota Associated with Low Fertility in Dairy Cows Using Endometrial Tissues Prior to First Artificial Insemination.Microbiol Spectr. 2023 Jun 15;11(3):e0476422. doi: 10.1128/spectrum.04764-22. Epub 2023 Apr 26. Microbiol Spectr. 2023. PMID: 37098918 Free PMC article.
-
BEEF SPECIES-RUMINANT NUTRITION CACTUS BEEF SYMPOSIUM: Influence of management decisions during heifer development on enhancing reproductive success and cow longevity1.J Anim Sci. 2019 Mar 1;97(3):1407-1414. doi: 10.1093/jas/sky440. J Anim Sci. 2019. PMID: 30462240 Free PMC article. Review.
-
[Reproduction of beef cattle].Tijdschr Diergeneeskd. 1992 Mar 1;117(5):133-8. Tijdschr Diergeneeskd. 1992. PMID: 1542865 Review. Dutch.
Cited by
-
Microbial Gatekeepers of Fertility in the Female Reproductive Microbiome of Cattle.Int J Mol Sci. 2024 Oct 10;25(20):10923. doi: 10.3390/ijms252010923. Int J Mol Sci. 2024. PMID: 39456706 Free PMC article. Review.
-
Holistic View and Novel Perspective on Ruminal and Extra-Gastrointestinal Methanogens in Cattle.Microorganisms. 2023 Nov 10;11(11):2746. doi: 10.3390/microorganisms11112746. Microorganisms. 2023. PMID: 38004757 Free PMC article. Review.
-
Optimizing bacteriophage screening and isolation methods for microbial samples derived from different body sites of cattle.bioRxiv [Preprint]. 2025 Jul 5:2025.07.04.663187. doi: 10.1101/2025.07.04.663187. bioRxiv. 2025. PMID: 40631329 Free PMC article. Preprint.
-
Reproductive Tract Microbiota of Mares.Vet Sci. 2024 Jul 18;11(7):324. doi: 10.3390/vetsci11070324. Vet Sci. 2024. PMID: 39058008 Free PMC article. Review.
-
Updates and Current Challenges in Reproductive Microbiome: A Comparative Analysis between Cows and Women.Animals (Basel). 2024 Jul 3;14(13):1971. doi: 10.3390/ani14131971. Animals (Basel). 2024. PMID: 38998083 Free PMC article. Review.
References
-
- Sheldon IM, Dobson H. 2003. Reproductive challenges facing the cattle industry at the beginning of the 21st century. Reprod 61:1–13. - PubMed
-
- Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, de Jonge JD, Poort L, Cuypers WJSS, Beckers NGM, Broekmans FJM, Cohlen BJ, den Hartog JE, Fleischer K, Lambalk CB, Smeenk JMJS, Budding AE, Laven JSE. 2019. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod 34:1042–1054. doi: 10.1093/humrep/dez065 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

