Feasibility of laparoscopic Visceral-Peritoneal Debulking (L-VPD) in patients with stage III-IV ovarian cancer: the ULTRA-LAP trial pilot study
- PMID: 37921599
- PMCID: PMC10948990
- DOI: 10.3802/jgo.2024.35.e14
Feasibility of laparoscopic Visceral-Peritoneal Debulking (L-VPD) in patients with stage III-IV ovarian cancer: the ULTRA-LAP trial pilot study
Abstract
Objective: A non-randomized prospective clinical trial (ULTRA-LAP) was registered to test safety, side effects and efficacy of laparoscopic Visceral-Peritoneal Debulking (L-VPD) in patients with stage III-IV ovarian cancer (OC). A pilot study was designed to identify which OC patients are suitable to undergo L-VPD.
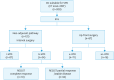
Methods: Between March 2016 and October 2021, all consecutive patients with OC underwent exploratory laparoscopy (EXL). All patients whose disease was deemed amenable for a complete resection (CR) at imaging review and EXL, underwent VPD. In all patients a consistent attempt was made at completing L-VPD.
Results: Two hundred and eight OC had EXL in the study period: 121 underwent interval VPD and 87 up-front VPD. Overall, 158 patients had VPD by laparotomy (75.9%) and 50 (24.1%) had L-VPD, of which 34 patients as interval (iL-VPD) and 16 as up-front (uL-VPD). Intra- and post-operative morbidity was very low in the L-VPD group. CR rate was 98% in L-VPD group and 94% in VPD. Most common reason for conversion was diaphragmatic disease extending dorsally.
Conclusion: In the pilot study of ULTRA-LAP, L-VPD was completed in 24,1% of OC. Initial analysis supports the feasibility of L-VPD in 2 groups of OC: those with no gross disease at interval surgery and those with gross visible disease at upfront or interval surgery, but limited to: pelvis (including recto-sigmoid), gastro colic omentum, peritoneum and diaphragm, the latter not requiring dorsal liver mobilization. Both groups had 100% feasibility and have been thus forth recruited to ULTRA-LAP.
Trial registration: ClinicalTrials.gov Identifier: NCT05862740.
Keywords: Laparoscopic Debulking; Minimally Invasive Debulking Surgery; Ovarian Cancer; Visceral-Peritoneal Debulking.
© 2024. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Colombo N, Sessa C, Bois AD, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019;30:672–705. - PubMed

