Improved Photoelectrochemical Performance of WO3/BiVO4 Heterojunction Photoanodes via WO3 Nanostructuring
- PMID: 37921705
- PMCID: PMC10658457
- DOI: 10.1021/acsami.3c10869
Improved Photoelectrochemical Performance of WO3/BiVO4 Heterojunction Photoanodes via WO3 Nanostructuring
Abstract
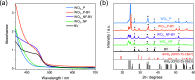
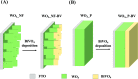
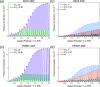
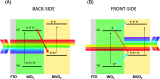
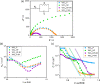
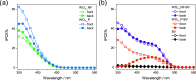
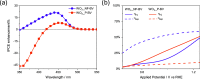
WO3/BiVO4 heterojunction photoanodes can be efficiently employed in photoelectrochemical (PEC) cells for the conversion of water into molecular oxygen, the kinetic bottleneck of water splitting. Composite WO3/BiVO4 photoelectrodes possessing a nanoflake-like morphology have been synthesized through a multistep process and their PEC performance was investigated in comparison to that of WO3/BiVO4 photoelectrodes displaying a planar surface morphology and similar absorption properties and thickness. PEC tests, also in the presence of a sacrificial hole scavenger, electrochemical impedance analysis under simulated solar irradiation, and incident photon to current efficiency measurements highlighted that charge transport and charge recombination issues affecting the performance of the planar composite can be successfully overcome by nanostructuring the WO3 underlayer in nanoflake-like WO3/BiVO4 heterojunction electrodes.
Keywords: bismuth vanadate; heterojunction; nanostructuring; photoanodes; tungsten trioxide; water oxidation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Maeda K.; Domen K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1 (18), 2655–2661. 10.1021/jz1007966. - DOI

