Use of Dexmedetomidine and Opioids in Hospitalized Preterm Infants
- PMID: 37921767
- PMCID: PMC10625033
- DOI: 10.1001/jamanetworkopen.2023.41033
Use of Dexmedetomidine and Opioids in Hospitalized Preterm Infants
Abstract
Importance: Dexmedetomidine, an α2-adrenergic agonist, is not approved by the Food and Drug Administration for use in premature infants. However, the off-label use of dexmedetomidine in premature infants has increased 50-fold in the past decade. Currently, there are no large studies characterizing dexmedetomidine use in US neonatal intensive care units (NICUs) or comparing the use of dexmedetomidine vs opioids in infants.
Objectives: To describe dexmedetomidine use patterns in the NICU and examine the association between dexmedetomidine and opioid use in premature infants.
Design, setting, and participants: A multicenter, observational cohort study was conducted from November 11, 2022, to April 4, 2023. Participants were inborn infants born between 22 weeks, 0 days, and 36 weeks, 6 days, of gestation at 1 of 383 Pediatrix Medical Group NICUs across the US between calendar years 2010 and 2020.
Main outcome and measure: Exposure to medications of interest defined as total days of exposure, timing of use, and changes over time.
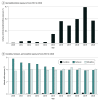
Results: A total of 395 122 infants were included in the analysis. Median gestational age was 34 (IQR, 32-35) weeks, and median birth weight was 2040 (IQR, 1606-2440) g. There were 384 infants (0.1% of total; 58.9% male) who received dexmedetomidine. Infants who received dexmedetomidine were born more immature, had lower birth weight, longer length of hospitalization, more opioid exposure, and more days of mechanical ventilation. Dexmedetomidine use increased from 0.003% in 2010 to 0.185% in 2020 (P < .001 for trend), while overall opioid exposure decreased from 8.5% in 2010 to 7.2% in 2020 (P < .001 for trend). The median postmenstrual age at first dexmedetomidine exposure was 31 (IQR, 27-36) weeks, and the median postnatal age at first dexmedetomidine exposure was 3 (IQR, 1-35) days. The median duration of dexmedetomidine receipt was 6 (IQR, 2-14) days.
Conclusion and relevance: The findings of this multicenter cohort study of premature infants suggest that dexmedetomidine use increased significantly between 2010 and 2020, while overall opioid exposure decreased. Future studies are required to further examine the short- and long-term effects of dexmedetomidine in premature and critically ill infants.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous