Effects of amyloid β (Aβ)42 and Gasdermin D on the progression of Alzheimer's disease in vitro and in vivo through the regulation of astrocyte pyroptosis
- PMID: 37921870
- PMCID: PMC10683627
- DOI: 10.18632/aging.205174
Effects of amyloid β (Aβ)42 and Gasdermin D on the progression of Alzheimer's disease in vitro and in vivo through the regulation of astrocyte pyroptosis
Abstract
Purpose: The study aimed to investigate whether astrocyte pyroptosis, and the subsequent neuroinflammatory response that exerts amyloid β (Aβ) neurotoxic effects, has an effect on endothelial cells, along with the underlying mechanisms.
Methods: In vivo, 5 μL of disease venom was injected into the lateral ventricle of APP/PS1 mice for treatment. Pyroptosis was induced by treating astrocytes with Aβ42 in vitro. Small interfering RNA (siRNA) was used to silence caspase-1 and Gasdermin D (GSDMD) mRNA expression. Cell viability was determined using a CCK-8 detection kit. Scanning electron microscopy (SEM), Annexin V/propidium iodide (PI) double staining, RT-qPCR, immunofluorescence, western blotting, and enzyme-linked immunosorbent assay (ELISA) were used to detect cell pyroptosis. The degree of pathological damage to the brain and aortic tissue was assessed by hematoxylin-eosin staining and immunohistochemistry.
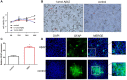
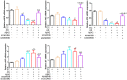
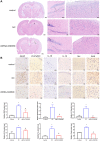
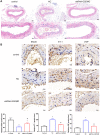
Results: Aβ42 induced astrocyte pyroptosis dependent on the GSDMD/Gasdermin E (GSDME)/Caspase 11/NLRP3 pathway, releasing large amounts of inflammatory factors, such as TNF-α, IL-1α, IL-1β, and IL-18. Astrocyte pyroptosis caused endothelial cell dysfunction and release of large amounts of vasoconstrictors (ET and vWF). Knockdown of GSDMD reduced astrocyte pyroptosis in the cerebral cortex and hippocampal tissue, decreased the release of inflammatory factors IL-1 β and IL-18, reduced Aβ deposition and tau protein, increased the release of peripheral vasodilator substances (eNOS), and decreased the release of vasoconstrictor substances (ET, vWF), thereby reducing brain tissue damage and vascular injury in APP/PS1 mice.
Conclusion: Aβ42 induced astrocyte pyroptosis, while GSDMD knockout inhibited astrocyte pyroptosis, reduced the release of inflammatory factors, and alleviated brain tissue damage and vascular damage in APP/PS1 mice. Therefore, GSDMD is a novel therapeutic target for Alzheimer's disease.
Purpose: The study aimed to investigate whether astrocyte pyroptosis, and the subsequent neuroinflammatory response that exerts amyloid β (Aβ) neurotoxic effects, has an effect on endothelial cells, along with the underlying mechanisms.
Methods: In vivo, 5 μL of disease venom was injected into the lateral ventricle of APP/PS1 mice for treatment. Pyroptosis was induced by treating astrocytes with Aβ42 in vitro. Small interfering RNA (siRNA) was used to silence caspase-1 and Gasdermin D (GSDMD) mRNA expression. Cell viability was determined using a CCK-8 detection kit. Scanning electron microscopy (SEM), Annexin V/propidium iodide (PI) double staining, RT-qPCR, immunofluorescence, western blotting, and enzyme-linked immunosorbent assay (ELISA) were used to detect cell pyroptosis. The degree of pathological damage to the brain and aortic tissue was assessed by hematoxylin-eosin staining and immunohistochemistry.
Results: Aβ42 induced astrocyte pyroptosis dependent on the GSDMD/Gasdermin E (GSDME)/Caspase 11/NLRP3 pathway, releasing large amounts of inflammatory factors, such as TNF-α, IL-1α, IL-1β, and IL-18. Astrocyte pyroptosis caused endothelial cell dysfunction and release of large amounts of vasoconstrictors (ET and vWF). Knockdown of GSDMD reduced astrocyte pyroptosis in the cerebral cortex and hippocampal tissue, decreased the release of inflammatory factors IL-1 β and IL-18, reduced Aβ deposition and tau protein, increased the release of peripheral vasodilator substances (eNOS), and decreased the release of vasoconstrictor substances (ET, vWF), thereby reducing brain tissue damage and vascular injury in APP/PS1 mice.
Conclusion: Aβ42 induced astrocyte pyroptosis, while GSDMD knockout inhibited astrocyte pyroptosis, reduced the release of inflammatory factors, and alleviated brain tissue damage and vascular damage in APP/PS1 mice. Therefore, GSDMD is a novel therapeutic target for Alzheimer's disease.
Keywords: Alzheimer’s disease; GSDMD; amyloid β; astrocyte pyroptosis; endothelial cell.
Conflict of interest statement
Figures


Similar articles
-
New mechanism of nerve injury in Alzheimer's disease: β-amyloid-induced neuronal pyroptosis.J Cell Mol Med. 2020 Jul;24(14):8078-8090. doi: 10.1111/jcmm.15439. Epub 2020 Jun 10. J Cell Mol Med. 2020. PMID: 32521573 Free PMC article.
-
Copper Overload Promotes β-amyloid Induced NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis in Alzheimer's Disease.J Integr Neurosci. 2024 Oct 21;23(10):194. doi: 10.31083/j.jin2310194. J Integr Neurosci. 2024. PMID: 39473154
-
Inhibiting NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis: A New Role of GADD45B's Role in Hypertriglyceridemia-Induced Acute Pancreatitis.Discov Med. 2025 Jun;37(197):1105-1116. doi: 10.24976/Discov.Med.202537197.98. Discov Med. 2025. PMID: 40485526
-
Perspectives on the mechanism of pyroptosis after intracerebral hemorrhage.Front Immunol. 2022 Sep 5;13:989503. doi: 10.3389/fimmu.2022.989503. eCollection 2022. Front Immunol. 2022. PMID: 36131917 Free PMC article. Review.
-
Pyroptosis in neutrophils: Multimodal integration of inflammasome and regulated cell death signaling pathways.Immunol Rev. 2023 Mar;314(1):229-249. doi: 10.1111/imr.13186. Epub 2023 Jan 19. Immunol Rev. 2023. PMID: 36656082 Free PMC article. Review.
Cited by
-
Identification of Molecular Correlations of GSDMD with Pyroptosis inAlzheimer's Disease.Comb Chem High Throughput Screen. 2024;27(14):2125-2139. doi: 10.2174/0113862073285497240226061936. Comb Chem High Throughput Screen. 2024. PMID: 39099451
-
NLRP3 inflammasome in Alzheimer's disease: molecular mechanisms and emerging therapies.Front Immunol. 2025 Apr 7;16:1583886. doi: 10.3389/fimmu.2025.1583886. eCollection 2025. Front Immunol. 2025. PMID: 40260242 Free PMC article. Review.
-
Pyroptosis: inflammatory cell death mechanism and its pathological roles in neurological diseases and injuries.Apoptosis. 2025 Aug 14. doi: 10.1007/s10495-025-02160-7. Online ahead of print. Apoptosis. 2025. PMID: 40813542 Review.
-
Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer's disease.Open Life Sci. 2025 Mar 18;20(1):20221043. doi: 10.1515/biol-2022-1043. eCollection 2025. Open Life Sci. 2025. PMID: 40109770 Free PMC article.
-
Targeting ceramide-induced microglial pyroptosis: Icariin is a promising therapy for Alzheimer's disease.J Pharm Anal. 2025 Apr;15(4):101106. doi: 10.1016/j.jpha.2024.101106. Epub 2024 Sep 19. J Pharm Anal. 2025. PMID: 40256246 Free PMC article.
References
-
- van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer's Disease-A Review. Adv Nutr. 2019; 10:1040–65. 10.1093/advances/nmz054 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

