Histo-Molecular Factors of Response to Combined Chemotherapy and Immunotherapy in Non-Small Cell Lung Cancers
- PMID: 37921939
- PMCID: PMC10663251
- DOI: 10.1007/s11523-023-01009-w
Histo-Molecular Factors of Response to Combined Chemotherapy and Immunotherapy in Non-Small Cell Lung Cancers
Abstract
Background: Chemo-immunotherapy (CIT) is the standard of care for advanced non-small cell lung cancer (NSCLC), but the impact of routinely available histo-molecular biomarkers on its efficacy has not yet been fully assessed.
Objective: The purpose of this multicenter study was to evaluate the clinical activity of CIT according to oncogenic drivers, STK11 and TP53 mutations, and MET overexpression.
Patients and methods: Patients receiving CIT for advanced NSCLC with available comprehensive molecular profile were included. The primary endpoint was progression-free survival (PFS), adjusted on main confounding factors, and secondary endpoints were overall survival (OS) and objective response rate.
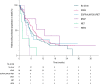
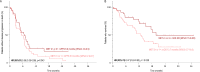
Results: Among the 195 patients included between September 2018 and October 2021, 88 (41%) had a KRAS mutation, 16 (8.2%) an EGFR mutation or an ALK, ROS1, or RET rearrangement, 11 (5.6%) a BRAF mutation, 6 (3.1%) a MET exon 14 mutation or MET amplification, and 5 (2.6%) a HER2 mutation. Seventy-seven patients (39.5%) had none of these alterations. The median PFS was 6.4 months (95% CI 5.3-7.3). Per subgroup, the median PFS was 7.1 months (5.4-8.9) for KRAS, 5.5 months (2.5-15.3) for EGFR/ALK/ROS1/RET, 12.9 months (2.6-not reached [NR]) for BRAF, 1.5 months (0.6-NR) for MET, 3.9 months (2.6-NR) for HER2, and 5.6 months (4.7-7.8) for patients without any oncogenic alteration. No difference in PFS was observed between the KRAS, BRAF, EGFR/ALK/ROS1/RET, and no-driver subgroups. STK11 mutations were associated with poor PFS (HR 1.59 [95% CI 1.01-2.51]) whereas TP53 mutations had no impact. MET overexpression was associated with longer PFS (HR 0.59 [95% CI 0.35-0.99]).
Conclusion: This study suggests that the efficacy of combining pembrolizumab with pemetrexed and platinum-based chemotherapy differs according to the histo-molecular biomarkers, which may help to identify patients liable to benefit from CIT.
© 2023. The Author(s).
Conflict of interest statement
A.B.C. has received research grants (paid to institution) from Merck; consulting fees from Novartis and Roche; a speaker honorarium from Astra-Zeneca, BMS, MSD, Pfizer, Novartis, Takeda, Janssen, Roche, Abbvie, Amgen, and Exeliom; support from Pfizer, Novartis, and MSD to attend meetings; and has served on advisory boards for Novartis and InhaTarget. V.L. has received a speaker honorarium from Novartis, Astra-Zeneca and MSD. E.D. has received a speaker honorarium from Novartis. M.M., H.B., N.P., S.B., S.H., F.E., and G.C. declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Figures


References
-
- Clinical Practice Living Guidelines—Metastatic Non-Small-Cell Lung Cancer | ESMO, (n.d.). https://www.esmo.org/guidelines/guidelines-by-topic/lung-and-chest-tumou.... Accessed 6 Aug 2022.
-
- de Castro G, Kudaba I, Wu Y-L, Lopes G, Kowalski DM, Turna HZ, et al. KEYNOTE-042 5-year survival update: pembrolizumab versus chemotherapy in patients with previously untreated, PD-L1–positive, locally advanced or metastatic non–small-cell lung cancer. J Immunother Cancer. 2021 doi: 10.1136/jitc-2021-SITC2021.363. - DOI
-
- Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized, phase III Trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39:723–733. doi: 10.1200/JCO.20.01605. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

