Netrin-1 feedforward mechanism promotes pancreatic cancer liver metastasis via hepatic stellate cell activation, retinoid, and ELF3 signaling
- PMID: 37922311
- PMCID: PMC12270551
- DOI: 10.1016/j.celrep.2023.113369
Netrin-1 feedforward mechanism promotes pancreatic cancer liver metastasis via hepatic stellate cell activation, retinoid, and ELF3 signaling
Abstract
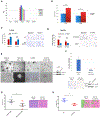
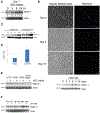
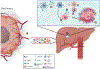
The biology of metastatic pancreatic ductal adenocarcinoma (PDAC) is distinct from that of the primary tumor due to changes in cell plasticity governed by a distinct transcriptome. Therapeutic strategies that target this distinct biology are needed. We detect an upregulation of the neuronal axon guidance molecule Netrin-1 in PDAC liver metastases that signals through its dependence receptor (DR), uncoordinated-5b (Unc5b), to facilitate metastasis in vitro and in vivo. The mechanism of Netrin-1 induction involves a feedforward loop whereby Netrin-1 on the surface of PDAC-secreted extracellular vesicles prepares the metastatic niche by inducing hepatic stellate cell activation and retinoic acid secretion that in turn upregulates Netrin-1 in disseminated tumor cells via RAR/RXR and Elf3 signaling. While this mechanism promotes PDAC liver metastasis, it also identifies a therapeutic vulnerability, as it can be targeted using anti-Netrin-1 therapy to inhibit metastasis using the Unc5b DR cell death mechanism.
Keywords: CP: Cancer; Elf3; Netrin-1; Unc5b; axon guidance; extracellular vesicles; hepatic stellate cells; metastasis; metastatic niche; pancreatic cancer; retinoic acid.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- McDonald OG, Li X, Saunders T, Tryggvadottir R, Mentch SJ, Warmoes MO, Word AE, Carrer A, Salz TH, Natsume S, et al. (2017). Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat. Genet 49, 367–376. 10.1038/ng.3753. - DOI - PMC - PubMed
-
- Pitarresi JR, Norgard RJ, Chiarella AM, Suzuki K, Bakir B, Sahu V, Li J, Zhao J, Marchand B, Wengyn MD, et al. (2021). PTHrP Drives Pancreatic Cancer Growth and Metastasis and Reveals a New Therapeutic Vulnerability. Cancer Discov. 11, 1774–1791. 10.1158/2159-8290.CD-20-1098. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

