Copper drives prion protein phase separation and modulates aggregation
- PMID: 37922348
- PMCID: PMC10624353
- DOI: 10.1126/sciadv.adi7347
Copper drives prion protein phase separation and modulates aggregation
Abstract
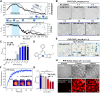
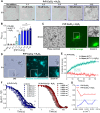
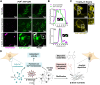
Prion diseases are characterized by prion protein (PrP) transmissible aggregation and neurodegeneration, which has been linked to oxidative stress. The physiological function of PrP seems related to sequestering of redox-active Cu2+, and Cu2+ dyshomeostasis is observed in prion disease brain. It is unclear whether Cu2+ contributes to PrP aggregation, recently shown to be mediated by PrP condensation. This study indicates that Cu2+ promotes PrP condensation in live cells at the cell surface and in vitro through copartitioning. Molecularly, Cu2+ inhibited PrP β-structure and hydrophobic residues exposure. Oxidation, induced by H2O2, triggered liquid-to-solid transition of PrP:Cu2+ condensates and promoted amyloid-like PrP aggregation. In cells, overexpression of PrPC initially protected against Cu2+ cytotoxicity but led to PrPC aggregation upon extended copper exposure. Our data suggest that PrP condensates function as a buffer for copper that prevents copper toxicity but can transition into PrP aggregation at prolonged oxidative stress.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

