Real-time liver motion estimation via deep learning-based angle-agnostic X-ray imaging
- PMID: 37922461
- PMCID: PMC10629841
- DOI: 10.1002/mp.16691
Real-time liver motion estimation via deep learning-based angle-agnostic X-ray imaging
Abstract
Background: Real-time liver imaging is challenged by the short imaging time (within hundreds of milliseconds) to meet the temporal constraint posted by rapid patient breathing, resulting in extreme under-sampling for desired 3D imaging. Deep learning (DL)-based real-time imaging/motion estimation techniques are emerging as promising solutions, which can use a single X-ray projection to estimate 3D moving liver volumes by solved deformable motion. However, such techniques were mostly developed for a specific, fixed X-ray projection angle, thereby impractical to verify and guide arc-based radiotherapy with continuous gantry rotation.
Purpose: To enable deformable motion estimation and 3D liver imaging from individual X-ray projections acquired at arbitrary X-ray scan angles, and to further improve the accuracy of single X-ray-driven motion estimation.
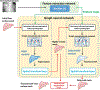
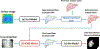
Methods: We developed a DL-based method, X360, to estimate the deformable motion of the liver boundary using an X-ray projection acquired at an arbitrary gantry angle (angle-agnostic). X360 incorporated patient-specific prior information from planning 4D-CTs to address the under-sampling issue, and adopted a deformation-driven approach to deform a prior liver surface mesh to new meshes that reflect real-time motion. The liver mesh motion is solved via motion-related image features encoded in the arbitrary-angle X-ray projection, and through a sequential combination of rigid and deformable registration modules. To achieve the angle agnosticism, a geometry-informed X-ray feature pooling layer was developed to allow X360 to extract angle-dependent image features for motion estimation. As a liver boundary motion solver, X360 was also combined with priorly-developed, DL-based optical surface imaging and biomechanical modeling techniques for intra-liver motion estimation and tumor localization.
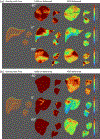
Results: With geometry-aware feature pooling, X360 can solve the liver boundary motion from an arbitrary-angle X-ray projection. Evaluated on a set of 10 liver patient cases, the mean (± s.d.) 95-percentile Hausdorff distance between the solved liver boundary and the "ground-truth" decreased from 10.9 (±4.5) mm (before motion estimation) to 5.5 (±1.9) mm (X360). When X360 was further integrated with surface imaging and biomechanical modeling for liver tumor localization, the mean (± s.d.) center-of-mass localization error of the liver tumors decreased from 9.4 (± 5.1) mm to 2.2 (± 1.7) mm.
Conclusion: X360 can achieve fast and robust liver boundary motion estimation from arbitrary-angle X-ray projections for real-time imaging guidance. Serving as a surface motion solver, X360 can be integrated into a combined framework to achieve accurate, real-time, and marker-less liver tumor localization.
Keywords: X-ray; graph neural network; liver; real-time imaging.
© 2023 American Association of Physicists in Medicine.
Conflict of interest statement
Conflict of Interest Statement
The authors have no relevant conflicts of interest to disclose.
Figures




Similar articles
-
A conditional point cloud diffusion model for deformable liver motion tracking via a single arbitrarily-angled x-ray projection.Phys Med Biol. 2025 Jun 25;70(12):125021. doi: 10.1088/1361-6560/addf0e. Phys Med Biol. 2025. PMID: 40446832 Free PMC article.
-
Real-time liver tumor localization via combined surface imaging and a single x-ray projection.Phys Med Biol. 2023 Mar 9;68(6):065002. doi: 10.1088/1361-6560/acb889. Phys Med Biol. 2023. PMID: 36731143 Free PMC article.
-
Automatic liver tumor localization using deep learning-based liver boundary motion estimation and biomechanical modeling (DL-Bio).Med Phys. 2021 Dec;48(12):7790-7805. doi: 10.1002/mp.15275. Epub 2021 Nov 19. Med Phys. 2021. PMID: 34632589 Free PMC article.
-
Advances in 4D medical imaging and 4D radiation therapy.Technol Cancer Res Treat. 2008 Feb;7(1):67-81. doi: 10.1177/153303460800700109. Technol Cancer Res Treat. 2008. PMID: 18198927 Review.
-
Deep learning-based target tracking with X-ray images for radiotherapy: a narrative review.Quant Imaging Med Surg. 2024 Mar 15;14(3):2671-2692. doi: 10.21037/qims-23-1489. Epub 2024 Mar 7. Quant Imaging Med Surg. 2024. PMID: 38545053 Free PMC article. Review.
Cited by
-
Real-time CBCT Imaging and Motion Tracking via a Single Arbitrarily-angled X-ray Projection by a Joint Dynamic Reconstruction and Motion Estimation (DREME) Framework.ArXiv [Preprint]. 2024 Sep 25:arXiv:2409.04614v2. ArXiv. 2024. Update in: Phys Med Biol. 2025 Jan 21;70(2). doi: 10.1088/1361-6560/ada519. PMID: 39398221 Free PMC article. Updated. Preprint.
-
Real-time CBCT imaging and motion tracking via a single arbitrarily-angled x-ray projection by a joint dynamic reconstruction and motion estimation (DREME) framework.Phys Med Biol. 2025 Jan 21;70(2):025026. doi: 10.1088/1361-6560/ada519. Phys Med Biol. 2025. PMID: 39746309 Free PMC article.
-
Deep learning-based estimation of respiration-induced deformation from surface motion: A proof-of-concept study on 4D thoracic image synthesis.Med Phys. 2025 Jul;52(7):e17804. doi: 10.1002/mp.17804. Epub 2025 Apr 5. Med Phys. 2025. PMID: 40186879 Free PMC article.
-
Dynamic CBCT imaging using prior model-free spatiotemporal implicit neural representation (PMF-STINR).Phys Med Biol. 2024 May 23;69(11):115030. doi: 10.1088/1361-6560/ad46dc. Phys Med Biol. 2024. PMID: 38697195 Free PMC article.
-
A conditional point cloud diffusion model for deformable liver motion tracking via a single arbitrarily-angled x-ray projection.Phys Med Biol. 2025 Jun 25;70(12):125021. doi: 10.1088/1361-6560/addf0e. Phys Med Biol. 2025. PMID: 40446832 Free PMC article.
References
-
- Verellen D, De Ridder M, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7(12):949–960. - PubMed
-
- Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4(9):737–747. - PubMed
-
- Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9(12):688–699. - PubMed
-
- Tubiana M, Eschwege F. Conformal radiotherapy and intensity-modulated radiotherapy--clinical data. Acta Oncol. 2000;39(5):555–567. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

