A CT-based radiomics classification model for the prediction of histological type and tumour grade in retroperitoneal sarcoma (RADSARC-R): a retrospective multicohort analysis
- PMID: 37922931
- PMCID: PMC10618402
- DOI: 10.1016/S1470-2045(23)00462-X
A CT-based radiomics classification model for the prediction of histological type and tumour grade in retroperitoneal sarcoma (RADSARC-R): a retrospective multicohort analysis
Abstract
Background: Retroperitoneal sarcomas are tumours with a poor prognosis. Upfront characterisation of the tumour is difficult, and under-grading is common. Radiomics has the potential to non-invasively characterise the so-called radiological phenotype of tumours. We aimed to develop and independently validate a CT-based radiomics classification model for the prediction of histological type and grade in retroperitoneal leiomyosarcoma and liposarcoma.
Methods: A retrospective discovery cohort was collated at our centre (Royal Marsden Hospital, London, UK) and an independent validation cohort comprising patients recruited in the phase 3 STRASS study of neoadjuvant radiotherapy in retroperitoneal sarcoma. Patients aged older than 18 years with confirmed primary leiomyosarcoma or liposarcoma proceeding to surgical resection with available contrast-enhanced CT scans were included. Using the discovery dataset, a CT-based radiomics workflow was developed, including manual delineation, sub-segmentation, feature extraction, and predictive model building. Separate probabilistic classifiers for the prediction of histological type and low versus intermediate or high grade tumour types were built and tested. Independent validation was then performed. The primary objective of the study was to develop radiomic classification models for the prediction of retroperitoneal leiomyosarcoma and liposarcoma type and histological grade.
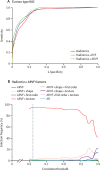
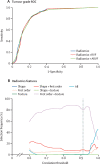
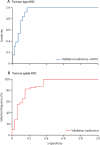
Findings: 170 patients recruited between Oct 30, 2016, and Dec 23, 2020, were eligible in the discovery cohort and 89 patients recruited between Jan 18, 2012, and April 10, 2017, were eligible in the validation cohort. In the discovery cohort, the median age was 63 years (range 27-89), with 83 (49%) female and 87 (51%) male patients. In the validation cohort, median age was 59 years (range 33-77), with 46 (52%) female and 43 (48%) male patients. The highest performing model for the prediction of histological type had an area under the receiver operator curve (AUROC) of 0·928 on validation, based on a feature set of radiomics and approximate radiomic volume fraction. The highest performing model for the prediction of histological grade had an AUROC of 0·882 on validation, based on a radiomics feature set.
Interpretation: Our validated radiomics model can predict the histological type and grade of retroperitoneal sarcomas with excellent performance. This could have important implications for improving diagnosis and risk stratification in retroperitoneal sarcomas.
Funding: Wellcome Trust, European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group, the National Institutes for Health, and the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AA reports funding from the Wellcome Trust paid to their institution. MRO, SD, and CM report funding paid to their institutions from the National Institute for Health and Care Research Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London. MRO reports funding from the Royal Marsden Cancer Charity to their institution. SD reports funding to their institution from Cancer Research UK. RH declares a previous role as the President of the Connective Tissue Oncology Society (in 2021). AG reports grants from PharmaMar and Nanobiotic; consulting fees from Novartis, Pfizer, Bayer, Lilly, PharmaMar, SpringWorks, and Boehringer Ingelheim; and honoraria from Deciphera. CPR reports royalties from UpTo Date. DVG reports grants from Elekta, IntraOp, and Orfit to their institution; consulting fees from Sanofi, Takeda, Novartis, and Merck; payment or honoraria from Elekta for lectures and support for attending meetings or travel; payment from Sanofi for expert testimony; and also declares a role in the Belgian College of Oncology and a research partnership with Elekta, Siemens, IntraOp and, MIM software, and a commercial partnership with Orfit, VisionRT, Philips, and Precisionxray. CLP reports payment from AstraZeneca to their institution for lectures; support for attending meetings and travel by Janseen and Ose Immunotherapeutics; and participation on advisory boards for AstraZeneca, Bristol Myers Squibbs and Varian. RJ reports grants from MSD and GSK and consulting fees from Adaptimmune, Astex, Athenex, Bayer, Boehringer Ingelheim, Blueprint, Clinigen, Eisai, Epizyme, Daichii, Deciphera, Immunedesign, Immunicum, Karma Oncology, Lilly, Merck, Mundipharma, PharmaMar, SpringWorks, Synox, Tracon, and UpTo Date. CM reports funding from European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group to their institution; payment or honoraria from TeleMedicine Clinic academy and GE Healthcare; and support for meetings from International Cancer Imaging Society. CM also declares a role as trustee of the British Institution of Radiology and is a Fellow of the International Cancer Imaging Society. All other authors declare no competing interests.
Figures
Comment in
-
Beyond the AJR: CT-Based Virtual Biopsy in Retroperitoneal Soft-Tissue Sarcomas.AJR Am J Roentgenol. 2024 Oct;223(4):e2430965. doi: 10.2214/AJR.24.30965. Epub 2024 Feb 28. AJR Am J Roentgenol. 2024. PMID: 38415577 No abstract available.
References
-
- Stojadinovic A, Yeh A, Brennan MF. Completely resected recurrent soft tissue sarcoma: primary anatomic site governs outcomes. J Am Coll Surg. 2002;194:436–447. - PubMed
-
- Schneider N, Strauss DC, Smith MJ, et al. The adequacy of core biopsy in the assessment of smooth muscle neoplasms of soft tissues: implications for treatment and prognosis. Am J Surg Pathol. 2017;41:923–931. - PubMed
-
- Bonvalot S, Gronchi A, Le Péchoux C, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21:1366–1377. - PubMed
-
- Crombé A, Fadli D, Italiano A, Saut O, Buy X, Kind M. Systematic review of sarcomas radiomics studies: bridging the gap between concepts and clinical applications? Eur J Radiol. 2020;132 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

