A framework for standardised tissue sampling and processing during resection of diffuse intracranial glioma: joint recommendations from four RANO groups
- PMID: 37922934
- PMCID: PMC10849105
- DOI: 10.1016/S1470-2045(23)00453-9
A framework for standardised tissue sampling and processing during resection of diffuse intracranial glioma: joint recommendations from four RANO groups
Abstract
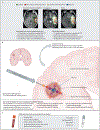
Surgical resection represents the standard of care for people with newly diagnosed diffuse gliomas, and the neuropathological and molecular profile of the resected tissue guides clinical management and forms the basis for research. The Response Assessment in Neuro-Oncology (RANO) consortium is an international, multidisciplinary effort that aims to standardise research practice in neuro-oncology. These recommendations represent a multidisciplinary consensus from the four RANO groups: RANO resect, RANO recurrent glioblastoma, RANO radiotherapy, and RANO/PET for a standardised workflow to achieve a representative tumour evaluation in a disease characterised by intratumoural heterogeneity, including recommendations on which tumour regions should be surgically sampled, how to define those regions on the basis of preoperative imaging, and the optimal sample volume. Practical recommendations for tissue sampling are given for people with low-grade and high-grade gliomas, as well as for people with newly diagnosed and recurrent disease. Sampling of liquid biopsies is also addressed. A standardised workflow for subsequent handling of the resected tissue is proposed to avoid information loss due to decreasing tissue quality or insufficient clinical information. The recommendations offer a framework for prospective biobanking studies.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MS declares consultancy for Bracco and honoraria from GE Healthcare and AuntMinnie. ELR declares honoraria from AbbVie, Adastra, Daiichi Sankyo, LEO Pharma, Seagen, and Tocagen. BME declares advisory board participation and consulting for Medicenna, MedQIA, Servier Pharmaceuticals, Siemens, Janssen Pharmaceuticals, Imaging Endpoints, Kazia, Chimerix, Sumitomo Dainippon Pharma Oncology, ImmunoGenesis, Ellipses Pharma, Monteris, Neosoma, Alpheus Medical, Sagimet Biosciences, Sapience Therapeutics, and the Global Coalition for Adaptive Research. MMK declares consultancy for Blue Earth Diagnostics and research grants from EpicentRx and Blue Earth Diagnostics. NLA declares research grants from Novocure and honoraria from Novartis and Telix. MPM declares consulting for Karyopharm Therapeutics, Mevion Medical Systems, ZappRx, Sapience Therapeutics, and Xoft; being part of the board of directors for Oncoceutics; ownership of WARF patent 14/934,27, Topical Vasoconstrictor Preparations and Methods for Protecting Cells During Cancer Chemotherapy and Radiotherapy; and stock at Chimerix. MvdB declares consultancy for Celgene, BMS, Agios, Boehringer, AbbVie, Bayer, Carthera, Nerviano, and Genenta. MW declares research grants from Quercis and Versameb; honoraria from Novocure and Bayer; and consulting or advisory roles for CureVac, Medac, Novartis, Orbus Therapeutics, Philogen, and Bayer. MAV declares indirect equity and patent royalty interests from Infuseon Therapeutics; honoraria from Chimerix and Midatech; and research grants from DeNovo Pharma, Oncosynergy, Infuseon, and Chimerix. J-CT declares research grants from Novocure and Munich Surgical Imaging; participating on an advisory board for AAA Novartis; and royalties from Springer Publisher. PK, GR, NG, JTH, OS, PNH, MM, LvB, RYH, SMC, and MSB declare no competing interests.
Figures