Accurate and Efficient SAXS/SANS Implementation Including Solvation Layer Effects Suitable for Molecular Simulations
- PMID: 37923304
- PMCID: PMC10687869
- DOI: 10.1021/acs.jctc.3c00864
Accurate and Efficient SAXS/SANS Implementation Including Solvation Layer Effects Suitable for Molecular Simulations
Abstract
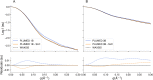
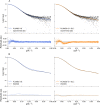
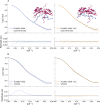
Small-angle X-ray and neutron scattering (SAXS/SANS) provide valuable insights into the structure and dynamics of biomolecules in solution, complementing a wide range of structural techniques, including molecular dynamics simulations. As contrast-based methods, they are sensitive not only to structural properties but also to solvent-solute interactions. Their use in molecular dynamics simulations requires a forward model that should be as fast and accurate as possible. In this work, we demonstrate the feasibility of calculating SAXS and SANS intensities using a coarse-grained representation consisting of one bead per amino acid and three beads per nucleic acid, with form factors that can be corrected on the fly to account for solvation effects at no additional computational cost. By coupling this forward model with molecular dynamics simulations restrained with SAS data, it is possible to determine conformational ensembles or refine the structure and dynamics of proteins and nucleic acids in agreement with the experimental results. To assess the robustness of this approach, we applied it to gelsolin, for which we acquired SAXS data on its closed state, and to a UP1-microRNA complex, for which we used previously collected measurements. Our hybrid-resolution small-angle scattering (hySAS) implementation, being distributed in PLUMED, can be used with atomistic and coarse-grained simulations using diverse restraining strategies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures