TAZ stimulates exercise-induced muscle satellite cell activation via Pard3-p38 MAPK-TAZ signalling axis
- PMID: 37923703
- PMCID: PMC10751443
- DOI: 10.1002/jcsm.13348
TAZ stimulates exercise-induced muscle satellite cell activation via Pard3-p38 MAPK-TAZ signalling axis
Abstract
Background: Exercise stimulates the activation of muscle satellite cells, which facilitate the maintenance of stem cells and their myogenic conversion during muscle regeneration. However, the underlying mechanism is not yet fully understood. This study shows that the transcriptional co-activator with PDZ-binding motif (TAZ) stimulates muscle regeneration via satellite cell activation.
Methods: Tazf/f mice were crossed with the paired box gene 7 (Pax7)creERT2 mice to generate muscle satellite cell-specific TAZ knockout (sKO) mice. Mice were trained in an endurance exercise programme for 4 weeks. Regenerated muscles were harvested and analysed by haematoxylin and eosin staining. Muscle tissues were also analysed by immunofluorescence staining, immunoblot analysis and quantitative reverse transcription PCR (qRT-PCR). For the in vitro study, muscle satellite cells from wild-type and sKO mice were isolated and analysed. Mitochondrial DNA was quantified by qRT-PCR using primers that amplify the cyclooxygenase-2 region of mitochondrial DNA. Quiescent and activated satellite cells were stained with MitoTracker Red CMXRos to analyse mitochondria. To study the p38 mitogen-activated protein kinase (MAPK)-TAZ signalling axis, p38 MAPK was activated by introducing the MAPK kinase 6 plasmid into satellite cells and also inhibited by treatment with the p38 MAPK inhibitor, SB203580.
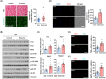
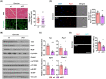
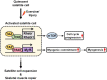
Results: TAZ interacts with Pax7 to induce Myf5 expression and stimulates mammalian target of rapamycin signalling for satellite cell activation. In sKO mice, TAZ depletion reduces muscle satellite cell number by 38% (0.29 ± 0.073 vs. 0.18 ± 0.034, P = 0.0082) and muscle regeneration. After muscle injury, TAZ levels (2.59-fold, P < 0.0001) increase in committed cells compared to self-renewing cells during asymmetric satellite cell division. Mechanistically, the polarity protein Pard3 induces TAZ (2.01-fold, P = 0.008) through p38 MAPK, demonstrating that the p38 MAPK-TAZ axis is important for muscle regeneration. Physiologically, endurance exercise training induces muscle satellite cell activation and increases muscle fibre diameter (1.33-fold, 43.21 ± 23.59 vs. 57.68 ± 23.26 μm, P = 0.0004) with increased TAZ levels (1.76-fold, P = 0.017). However, sKO mice had a 39% reduction in muscle satellite cell number (0.20 ± 0.03 vs. 0.12 ± 0.02, P = 0.0013) and 24% reduction in muscle fibre diameter compared to wild-type mice (61.07 ± 23.33 vs. 46.60 ± 24.29 μm, P = 0.0006).
Conclusions: Our results demonstrate a novel mechanism of TAZ-induced satellite cell activation after muscle injury and exercise, suggesting that activation of TAZ in satellite cells may ameliorate the muscle ageing phenotype and may be an important target protein for the drug development in sarcopenia.
Keywords: exercise; muscle regeneration; muscle satellite cell; sarcopenia.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Chang NC, Rudnicki MA. Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol 2014;107:161–181. - PubMed
-
- Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol 2011;13:127–133. - PubMed
-
- Bischoff R, Heintz C. Enhancement of skeletal muscle regeneration. Dev Dyn 1994;201:41–54. - PubMed
-
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf‐5 is required for the formation of skeletal muscle. Cell 1993;75:1351–1359. - PubMed
-
- Cornelison DD, Wold BJ. Single‐cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 1997;191:270–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

