Expression of G protein-coupled receptor GPR19 in normal and neoplastic human tissues
- PMID: 37923782
- PMCID: PMC10624815
- DOI: 10.1038/s41598-023-46395-3
Expression of G protein-coupled receptor GPR19 in normal and neoplastic human tissues
Abstract
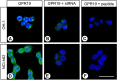
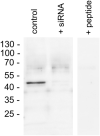
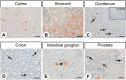
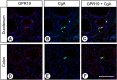
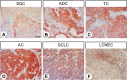
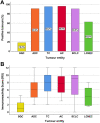
Little is known about the expression of the orphan G protein-coupled receptor GPR19 at the protein level. Therefore, we developed a rabbit antibody, targeting human GPR19. After verification of the antibody specificity using GPR19-expressing cell lines and a GPR19-specific siRNA, the antibody was used for immunohistochemical staining of a variety of formalin-fixed, paraffin-embedded normal and neoplastic human tissue samples. In normal tissues, GPR19 expression was detected in a distinct cell population within the cortex, in single cells of the pancreatic islets, in intestinal ganglia, gastric chief cells, and in endocrine cells of the bronchial tract, the gastrointestinal tract, and the prostate. Among the 30 different tumour entities investigated, strong GPR19 expression was found in adenocarcinomas, typical and atypical carcinoids of the lung, and small cell lung cancer. To a lesser extent, the receptor was also present in large cell neuroendocrine carcinomas of the lung, medullary thyroid carcinomas, parathyroid adenomas, pheochromocytomas, and a subpopulation of pancreatic neuroendocrine neoplasms. In lung tumours, a negative correlation with the expression of the proliferation marker Ki-67 and a positive interrelationship with patient survival was observed. Overall, our results indicate that in adenocarcinomas and neuroendocrine tumours of the lung GPR19 may serve as a suitable diagnostic or therapeutic target.
© 2023. The Author(s).
Conflict of interest statement
Daniel Kaemmerer received support for travelling to meetings by the companies IPSEN and PFIZER. Stefan Schulz is the founder and scientific advisor of 7TM Antibodies GmbH, Jena, Germany, and declares no competing non-financial interests but competing financial interests. All other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


References
-
- Yang D, Zhou Q, Labroska V, Qin S, Darbalaei S, Wu Y, Yuliantie E, Xie L, Tao H, Cheng J, Liu Q, Zhao S, Shui W, Jiang Y, Wang MW. G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 2021;6:7. doi: 10.1038/s41392-020-00435-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

