Glutathione Transferase Photoaffinity Labeling Displays GST Induction by Safeners and Pathogen Infection
- PMID: 37924215
- PMCID: PMC10799724
- DOI: 10.1093/pcp/pcad132
Glutathione Transferase Photoaffinity Labeling Displays GST Induction by Safeners and Pathogen Infection
Abstract
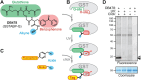
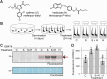
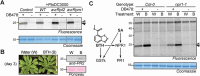
Glutathione transferases (GSTs) represent a large and diverse enzyme family involved in the detoxification of small molecules by glutathione conjugation in crops, weeds and model plants. In this study, we introduce an easy and quick assay for photoaffinity labeling of GSTs to study GSTs globally in various plant species. The small-molecule probe contains glutathione, a photoreactive group and a minitag for coupling to reporter tags via click chemistry. Under UV irradiation, this probe quickly and robustly labels GSTs in crude protein extracts of different plant species. Purification and mass spectrometry (MS) analysis of labeled proteins from Arabidopsis identified 10 enriched GSTs from the Phi(F) and Tau(U) classes. Photoaffinity labeling of GSTs demonstrated GST induction in wheat seedlings upon treatment with safeners and in Arabidopsis leaves upon infection with avirulent bacteria. Treatment of Arabidopsis with salicylic acid (SA) analog benzothiadiazole (BTH) induces GST labeling independent of NPR1, the master regulator of SA. Six Phi- and Tau-class GSTs that are induced upon BTH treatment were identified, and their labeling was confirmed upon transient overexpression. These data demonstrate that GST photoaffinity labeling is a useful approach to studying GST induction in crude extracts of different plant species upon different types of stress.
Keywords: Agrochemical; Chemical proteomics; GST; Glutathione transferase; Immunity; Photoaffinity labeling; Safener.
© The Author(s) 2023. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures




Similar articles
-
Proteomic analysis of glutathione S -transferases of Arabidopsis thaliana reveals differential salicylic acid-induced expression of the plant-specific phi and tau classes.Plant Mol Biol. 2004 Jan;54(2):205-19. doi: 10.1023/B:PLAN.0000028786.57439.b3. Plant Mol Biol. 2004. PMID: 15159623
-
Induction of glutathione S-transferases in Arabidopsis by herbicide safeners.Plant Physiol. 2002 Nov;130(3):1497-505. doi: 10.1104/pp.010066. Plant Physiol. 2002. PMID: 12428014 Free PMC article.
-
The rapid induction of glutathione S-transferases AtGSTF2 and AtGSTF6 by avirulent Pseudomonas syringae is the result of combined salicylic acid and ethylene signaling.Plant Cell Physiol. 2003 Jul;44(7):750-7. doi: 10.1093/pcp/pcg093. Plant Cell Physiol. 2003. PMID: 12881503
-
Plant glutathione transferases.Genome Biol. 2002;3(3):REVIEWS3004. doi: 10.1186/gb-2002-3-3-reviews3004. Epub 2002 Feb 26. Genome Biol. 2002. PMID: 11897031 Free PMC article. Review.
-
Glutathione S-Transferase Enzymes in Plant-Pathogen Interactions.Front Plant Sci. 2018 Dec 21;9:1836. doi: 10.3389/fpls.2018.01836. eCollection 2018. Front Plant Sci. 2018. PMID: 30622544 Free PMC article. Review.
References
-
- Bally J., Jung H., Mortimer C., Naim F., Philips J.G., Hellens R., et al. (2018) The rise and rise of Nicotiana benthamiana: a plant for all reasons. Annu. Rev. Phytopathol. 56: 405–426. - PubMed
-
- Basantani M. and Srivastava A. (2007) Plant glutathione transferases—a decade falls short. Can. J. Bot. 85: 443–456.
-
- Behringer C., Bartsch K. and Schaller A. (2011) Safeners recruit multiple signalling pathways for the orchestrated induction of the cellular xenobiotic detoxification machinery in Arabidopsis. Plant Cell Environ. 34: 1970–1985. - PubMed
-
- Blanco F., Garretón V., Frey N., Dominguez C., Pérez-Acle T., Van der Straeten D., et al. (2005) Identification of NPR1-dependent and independent genes early induced by salicylic acid treatment in Arabidopsis. Plant Mol. Biol. 59: 927–944. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

