Protocol for assembling and operating bipolar membrane water electrolyzers
- PMID: 37924520
- PMCID: PMC10656253
- DOI: 10.1016/j.xpro.2023.102606
Protocol for assembling and operating bipolar membrane water electrolyzers
Abstract
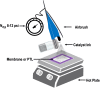
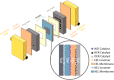
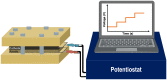
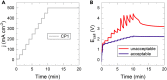
Renewable energy-driven bipolar membrane water electrolyzers (BPMWEs) are a promising technology for sustainable production of hydrogen from seawater and other impure water sources. Here, we present a protocol for assembling BPMWEs and operating them in a range of water feedstocks, including ultra-pure deionized water and seawater. We describe steps for membrane electrode assembly preparation, electrolyzer assembly, and electrochemical evaluation. For complete details on the use and execution of this protocol, please refer to Marin et al. (2023).1.
Keywords: Chemistry; Energy; Material Sciences.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have patents submitted and issued (US patent # 11,268,200) related to the content of this manuscript.
Figures











References
-
- Marin D.H., Perryman J.T., Hubert M.A., Lindquist G.A., Chen L., Aleman A.M., Kamat G.A., Niemann V.A., Stevens M.B., Regmi Y.N., et al. Hydrogen production with seawater-resilient bipolar membrane electrolyzers. Joule. 2023;7:765–781. doi: 10.1016/j.joule.2023.03.005. - DOI
-
- Salvatore D.A., Gabardo C.M., Reyes A., O’Brien C.P., Holdcroft S., Pintauro P., Bahar B., Hickner M., Bae C., Sinton D., et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy. 2021;6:339–348. doi: 10.1038/s41560-020-00761-x. - DOI
-
- Pärnamäe R., Mareev S., Nikonenko V., Melnikov S., Sheldeshov N., Zabolotskii V., Hamelers H.V.M., Tedesco M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021;617 doi: 10.1016/j.memsci.2020.118538. - DOI
-
- Wang X., Rossi R., Yan Z., Yang W., Hickner M.A., Mallouk T.E., Logan B.E. Balancing Water Dissociation and Current Densities To Enable Sustainable Hydrogen Production with Bipolar Membranes in Microbial Electrolysis Cells. Environ. Sci. Technol. 2019;53:14761–14768. doi: 10.1021/acs.est.9b05024. - DOI - PubMed

