Non-steroidal mineralocorticoid receptor antagonist finerenone ameliorates mitochondrial dysfunction via PI3K/Akt/eNOS signaling pathway in diabetic tubulopathy
- PMID: 37924663
- PMCID: PMC10661120
- DOI: 10.1016/j.redox.2023.102946
Non-steroidal mineralocorticoid receptor antagonist finerenone ameliorates mitochondrial dysfunction via PI3K/Akt/eNOS signaling pathway in diabetic tubulopathy
Abstract
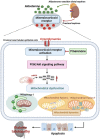
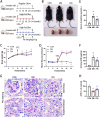
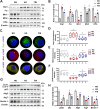
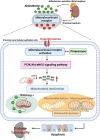
Diabetic tubulopathy (DT) is a recently recognized key pathology of diabetic kidney disease (DKD). The mitochondria-centric view of DT is emerging as a vital pathological factor in different types of metabolic diseases, such as DKD. Finerenone (FIN), a novel non-steroidal mineralocorticoid receptor antagonist, attenuates kidney inflammation and fibrosis in DKD, but the precise pathomechanisms remain unclear. The role of mineralocorticoid receptor (MR) in perturbing mitochondrial function via the PI3K/Akt/eNOS signaling pathway, including mitochondrial dynamics and mitophagy, was investigated under a diabetic state and high glucose (HG) ambiance. To elucidate how the activation of MR provokes mitochondrial dysfunction in DT, human kidney proximal tubular epithelial (HK-2) cells were exposed to HG, and then mitochondrial dynamics, mitophagy, mitochondrial ROS (mitoROS), signaling molecules PI3K, Akt, Akt phosphorylation and eNOS were probed. The above molecules or proteins were also explored in the kidneys of diabetic and FIN-treated mice. FIN treatment reduced oxidative stress, mitochondrial fragmentation, and apoptosis while restoring the mitophagy via PI3K/Akt/eNOS signaling pathway in HK-2 cells exposed to HG ambiance and tubular cells of DM mice. These findings linked MR activation to mitochondrial dysfunction via PI3K/Akt/eNOS signaling pathway in DT and highlight a pivotal but previously undiscovered role of FIN in alleviating renal tubule injury for the treatment of DKD.
Keywords: Diabetic kidney disease; Diabetic tubulopathy; Finerenone; Nonsteroidal mineralocorticoid receptor antagonist; PI3K/Akt/eNOS signaling pathway.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None declared.
Figures




References
-
- Wheeler D.C., et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:22–31. doi: 10.1016/s2213-8587(20)30369-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

