Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis
- PMID: 37924812
- PMCID: PMC10872579
- DOI: 10.1016/j.immuni.2023.10.004
Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis
Abstract
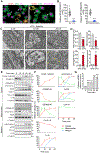
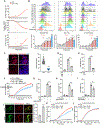
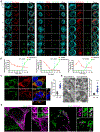
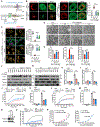
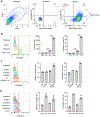
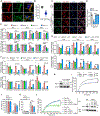
Gasdermin D (GSDMD)-activated inflammatory cell death (pyroptosis) causes mitochondrial damage, but its underlying mechanism and functional consequences are largely unknown. Here, we show that the N-terminal pore-forming GSDMD fragment (GSDMD-NT) rapidly damaged both inner and outer mitochondrial membranes (OMMs) leading to reduced mitochondrial numbers, mitophagy, ROS, loss of transmembrane potential, attenuated oxidative phosphorylation (OXPHOS), and release of mitochondrial proteins and DNA from the matrix and intermembrane space. Mitochondrial damage occurred as soon as GSDMD was cleaved prior to plasma membrane damage. Mitochondrial damage was independent of the B-cell lymphoma 2 family and depended on GSDMD-NT binding to cardiolipin. Canonical and noncanonical inflammasome activation of mitochondrial damage, pyroptosis, and inflammatory cytokine release were suppressed by genetic ablation of cardiolipin synthase (Crls1) or the scramblase (Plscr3) that transfers cardiolipin to the OMM. Phospholipid scramblase-3 (PLSCR3) deficiency in a tumor compromised pyroptosis-triggered anti-tumor immunity. Thus, mitochondrial damage plays a critical role in pyroptosis.
Keywords: CRLS1; GSDMD; IL-1; PLSCR3; cardiolipin; mitochondria; pyroptosis.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.L. and H.W. are cofounders and advisors of Ventus Therapeutics. J.C.K. consults and holds equity in Corner Therapeutics, Larkspur Biosciences, and Neumora Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

