MUC1-C Is a Common Driver of Acquired Osimertinib Resistance in NSCLC
- PMID: 37924972
- PMCID: PMC10939926
- DOI: 10.1016/j.jtho.2023.10.017
MUC1-C Is a Common Driver of Acquired Osimertinib Resistance in NSCLC
Erratum in
-
Corrigendum to 'MUC1-C Is a Common Driver of Acquired Osimertinib Resistance in NSCLC' [Journal of Thoracic Oncology 19 Issue 3 (2024) 434-450].J Thorac Oncol. 2025 Mar;20(3):399. doi: 10.1016/j.jtho.2024.12.004. Epub 2024 Dec 18. J Thorac Oncol. 2025. PMID: 39692639 No abstract available.
Abstract
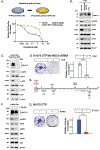
Introduction: Osimertinib is an irreversible EGFR tyrosine kinase inhibitor approved for the first-line treatment of patients with metastatic NSCLC harboring EGFR exon 19 deletions or L858R mutations. Patients treated with osimertinib invariably develop acquired resistance by mechanisms involving additional EGFR mutations, MET amplification, and other pathways. There is no known involvement of the oncogenic MUC1-C protein in acquired osimertinib resistance.
Methods: H1975/EGFR (L858R/T790M) and patient-derived NSCLC cells with acquired osimertinib resistance were investigated for MUC1-C dependence in studies of EGFR pathway activation, clonogenicity, and self-renewal capacity.
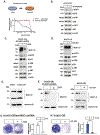
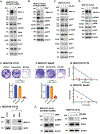
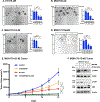
Results: We reveal that MUC1-C is up-regulated in H1975 osimertinib drug-tolerant persister cells and is necessary for activation of the EGFR pathway. H1975 cells selected for stable osimertinib resistance (H1975-OR) and MGH700-2D cells isolated from a patient with acquired osimertinib resistance are found to be dependent on MUC1-C for induction of (1) phospho (p)-EGFR, p-ERK, and p-AKT, (2) EMT, and (3) the resistant phenotype. We report that MUC1-C is also required for p-EGFR, p-ERK, and p-AKT activation and self-renewal capacity in acquired osimertinib-resistant (1) MET-amplified MGH170-1D #2 cells and (2) MGH121 Res#2/EGFR (T790M/C797S) cells. Importantly, targeting MUC1-C in these diverse models reverses osimertinib resistance. In support of these results, high MUC1 mRNA and MUC1-C protein expression is associated with a poor prognosis for patients with EGFR-mutant NSCLCs.
Conclusions: Our findings reveal that MUC1-C is a common effector of osimertinib resistance and is a potential target for the treatment of osimertinib-resistant NSCLCs.
Keywords: EGFR; MUC1-C; NSCLC; Osimertinib; Resistance.
Copyright © 2023 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
MUC1-C: The Occam Razor of Osimertinib Resistance?J Thorac Oncol. 2024 Mar;19(3):370-372. doi: 10.1016/j.jtho.2023.12.014. J Thorac Oncol. 2024. PMID: 38453323 No abstract available.
References
-
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169–181. - PubMed
-
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528–538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

