Naturally occurring canine laminopathy leading to a dilated and fibrosing cardiomyopathy in the Nova Scotia Duck Tolling Retriever
- PMID: 37925523
- PMCID: PMC10625583
- DOI: 10.1038/s41598-023-46601-2
Naturally occurring canine laminopathy leading to a dilated and fibrosing cardiomyopathy in the Nova Scotia Duck Tolling Retriever
Abstract
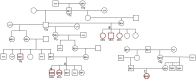
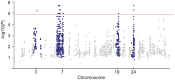
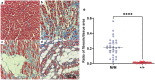
Dilated cardiomyopathy (DCM) is characterized by decreased systolic function and dilation of one or both ventricles, often leading to heart failure or sudden death. Two 10-month-old sibling Nova Scotia Duck Tolling Retrievers (NSDTR) died acutely with evidence of dilated cardiomyopathy with myocardial fibrosis. Association analysis using two cases and 35 controls identified three candidate regions homozygous in the two cases. Whole genome sequencing identified a frameshift deletion in the LMNA gene (NC_049228.1:g.41688530del, NP_001274080:p.(Asp576ThrfsTer124)). Three retrospectively identified NSDTRs with sudden death before 2 years of age and severe myocardial fibrosis were also homozygous for the deletion. One 5 year old with sudden death and myocardial fibrosis was heterozygous for the deletion. This variant was not identified in 722 dogs of other breeds, nor was it identified to be homozygous in 784 NSDTR. LMNA codes for lamin A/C proteins, which are type V intermediate filaments that provide structural support to the nuclear membrane. In humans, LMNA variants can cause DCM with sudden death as well as diseases of striated muscles, lipodystrophy, neuropathies, and accelerated aging disorders. This frameshift deletion is predicted to affect processing of prelamin A into lamin A. Pedigree analysis in the NSDTR and functional evaluation of heterozygotes is consistent with a predominantly recessive mode of inheritance and possibly low penetrance in heterozygotes in contrast to people, where most pathogenic LMNA variants are dominantly inherited.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures