Treosulfan vs busulfan conditioning for allogeneic bmt in children with nonmalignant disease: a randomized phase 2 trial
- PMID: 37925531
- PMCID: PMC10781637
- DOI: 10.1038/s41409-023-02135-9
Treosulfan vs busulfan conditioning for allogeneic bmt in children with nonmalignant disease: a randomized phase 2 trial
Abstract
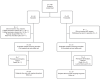
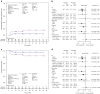
Optimal conditioning prior to allogeneic hematopoietic stem cell transplantation for children with non-malignant diseases is subject of ongoing research. This prospective, randomized, phase 2 trial compared safety and efficacy of busulfan with treosulfan based preparative regimens. Children with non-malignant diseases received fludarabine and either intravenous (IV) busulfan (4.8 to 3.2 mg/kg/day) or IV treosulfan (10, 12, or 14 g/m2/day). Thiotepa administration (2 × 5 mg/kg) was at the investigator's discretion. Primary endpoint was freedom from transplantation (treatment)-related mortality (freedom from TRM), defined as death between Days -7 and +100. Overall, 101 patients (busulfan 50, treosulfan 51) with at least 12 months follow-up were analyzed. Freedom from TRM was 90.0% (95% CI: 78.2%, 96.7%) after busulfan and 100.0% (95% CI: 93.0%, 100.0%) after treosulfan. Secondary outcomes (transplantation-related mortality [12.0% versus 3.9%]) and overall survival (88.0% versus 96.1%) favored treosulfan. Graft failure was more common after treosulfan (n = 11), than after busulfan (n = 2) while all patients were rescued by second procedures except one busulfan patient. CTCAE Grade III adverse events were similar in both groups. This study confirmed treosulfan to be an excellent alternative to busulfan and can be safely used for conditioning treatment in children with non-malignant disease.
© 2023. The Author(s).
Conflict of interest statement
K-WS: speaker fees Jazz, research and travel grants medac, travel grant Neovii. RB: research and travel grants medac, travel grant Neovii. PB: no relevant disclosures. JS: no relevant disclosures. Ansgar Schulz: no relevant disclosures. JG: no relevant disclosures. JG: no relevant disclosures. DR: no relevant disclosures. JS: no relevant disclosures. FF: no relevant disclosures. BG: Honoraria: Amgen GmbH, EUSA Pharma GmbH, medac GmbH, Novartis Pharma GmbH; Membership of advisory committee of Amgen GmbH, EUSA Pharma GmbH. FL: no relevant disclosures. Simona Piras: no relevant disclosures. PS: no relevant disclosures. SB: no relevant disclosures. MZ: no relevant disclosures. MC: no relevant disclosures. SC: no relevant disclosures. XL: employee of medac GmbH. JB: employee of medac GmbH. JK: employee of medac GmbH. MM: no relevant disclosures. KK: Speaker’s bureau: JazzPharma, medac, Novartis.
Figures